Amiloride Reduces Urokinase/Plasminogen-Driven Intratubular Complement Activation in Glomerular Proteinuria
- PMID: 38254266
- PMCID: PMC11000727
- DOI: 10.1681/ASN.0000000000000312
Amiloride Reduces Urokinase/Plasminogen-Driven Intratubular Complement Activation in Glomerular Proteinuria
Abstract
Significance statement: Proteinuria predicts accelerated decline in kidney function in CKD. The pathologic mechanisms are not well known, but aberrantly filtered proteins with enzymatic activity might be involved. The urokinase-type plasminogen activator (uPA)-plasminogen cascade activates complement and generates C3a and C5a in vitro / ex vivo in urine from healthy persons when exogenous, inactive, plasminogen, and complement factors are added. Amiloride inhibits uPA and attenuates complement activation in vitro and in vivo . In conditional podocin knockout (KO) mice with severe proteinuria, blocking of uPA with monoclonal antibodies significantly reduces the urine excretion of C3a and C5a and lowers tissue NLRP3-inflammasome protein without major changes in early fibrosis markers. This mechanism provides a link to proinflammatory signaling in proteinuria with possible long-term consequences for kidney function.
Background: Persistent proteinuria is associated with tubular interstitial inflammation and predicts progressive kidney injury. In proteinuria, plasminogen is aberrantly filtered and activated by urokinase-type plasminogen activator (uPA), which promotes kidney fibrosis. We hypothesized that plasmin activates filtered complement factors C3 and C5 directly in tubular fluid, generating anaphylatoxins, and that this is attenuated by amiloride, an off-target uPA inhibitor.
Methods: Purified C3, C5, plasminogen, urokinase, and urine from healthy humans were used for in vitro / ex vivo studies. Complement activation was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting, and ELISA. Urine and plasma from patients with diabetic nephropathy treated with high-dose amiloride and from mice with proteinuria (podocin knockout [KO]) treated with amiloride or inhibitory anti-uPA antibodies were analyzed.
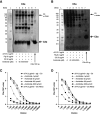
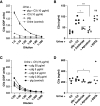
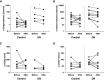
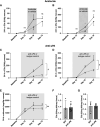
Results: The combination of uPA and plasminogen generated anaphylatoxins C3a and C5a from intact C3 and C5 and was inhibited by amiloride. Addition of exogenous plasminogen was sufficient for urine from healthy humans to activate complement. Conditional podocin KO in mice led to severe proteinuria and C3a and C5a urine excretion, which was attenuated reversibly by amiloride treatment for 4 days and reduced by >50% by inhibitory anti-uPA antibodies without altering proteinuria. NOD-, LRR- and pyrin domain-containing protein 3-inflammasome protein was reduced with no concomitant effect on fibrosis. In patients with diabetic nephropathy, amiloride reduced urinary excretion of C3dg and sC5b-9 significantly.
Conclusions: In conditions with proteinuria, uPA-plasmin generates anaphylatoxins in tubular fluid and promotes downstream complement activation sensitive to amiloride. This mechanism links proteinuria to intratubular proinflammatory signaling. In perspective, amiloride could exert reno-protective effects beyond natriuresis and BP reduction.
Clinical trial registry name and registration number: Increased Activity of a Renal Salt Transporter (ENaC) in Diabetic Kidney Disease, NCT01918488 and Increased Activity of ENaC in Proteinuric Kidney Transplant Recipients, NCT03036748 .
Copyright © 2024 by the American Society of Nephrology.
Conflict of interest statement
H. Andersen reports employment with EFKT. H. Birn reports consultancy for AstraZeneca, Boehringer Ingelheim, Bayer, Galapagos, and GlaxoSmithKline (GSK); research funding from GlaxoSmithKline (GSK) and Vifor Pharma; honoraria from AstraZeneca; a patent application under review and not filed at this point; and advisory or leadership role as President of the Danish Society of Nephrology and a member of working groups under the Danish Health Authority. C. Bistrup reports employment with Odense University Hospital, Odense, Denmark and University of Southern Denmark, Odense, Denmark. J.E. Henriksen reports employment with Odense University Hospital, Denmark. G.R. Hinrichs and B.L. Jensen report employment with University of Southern Denmark. I.K. Lund reports employment with and ownership interest in Novo Nordisk A/S. K. Weyer reports employment with Draupnir Bio; consultancy for Astex Pharmaceuticals, Muna Therapeutics, Novo Holdings, and Novo Nordisk Foundation; ownership interest in Denali Therapeutics, Draupnir Bio, Muna Therapeutics, Novo Nordisk, Teitur Trophics, and Verve Therapeutics; research funding from Novo Nordisk; patents or royalties from Aarhus University, Draupnir Bio, and Teitur Trophics; advisory or leadership role for Draupnir Bio; and other interests or relationships with Aarhus Lifescience and Biotech Alliance (ALBA) and Danish Nephrology Society. All remaining authors have nothing to disclose.
Figures



References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

