The relation between BTK expression and iron accumulation of myeloid cells in multiple sclerosis
- PMID: 38254312
- PMCID: PMC11328345
- DOI: 10.1111/bpa.13240
The relation between BTK expression and iron accumulation of myeloid cells in multiple sclerosis
Abstract
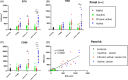
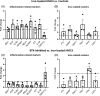
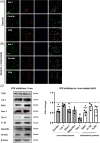
Activation of Bruton's tyrosine kinase (BTK) has been shown to play a crucial role in the proinflammatory response of B cells and myeloid cells upon engagement with B cell, Fc, Toll-like receptor, and distinct chemokine receptors. Previous reports suggest BTK actively contributes to the pathogenesis of multiple sclerosis (MS). The BTK inhibitor Evobrutinib has been shown to reduce the numbers of gadolinium-enhancing lesions and relapses in relapsing-remitting MS patients. In vitro, BTK inhibition resulted in reduced phagocytic activity and modulated BTK-dependent inflammatory signaling of microglia and macrophages. Here, we investigated the protein expression of BTK and CD68 as well as iron accumulation in postmortem control (n = 10) and MS (n = 23) brain tissue, focusing on microglia and macrophages. MS cases encompassed active, chronic active, and inactive lesions. BTK+ and iron+ cells positively correlated across all regions of interests and, along with CD68, revealed highest numbers in the center of active and at the rim of chronic active lesions. We then studied the effect of BTK inhibition in the human immortalized microglia-like HMC3 cell line in vitro. In particular, we loaded HMC3 cells with iron-dextran and subsequently administered the BTK inhibitor Evobrutinib. Iron treatment alone induced a proinflammatory phenotype and increased the expression of iron importers as well as the intracellular iron storage protein ferritin light chain (FTL). BTK inhibition of iron-laden cells dampened the expression of microglia-related inflammatory genes as well as iron-importers, whereas the iron-exporter ferroportin was upregulated. Our data suggest that BTK inhibition not only dampens the proinflammatory response but also reduces iron import and storage in activated microglia and macrophages with possible implications on microglial iron accumulation in chronic active lesions in MS.
Keywords: Bruton's tyrosine kinase; Evobrutinib; microglia; multiple sclerosis; myeloid cells; neuroinflammation.
© 2024 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Figures



References
-
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. 10.1016/S0002-9440(10)64537-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

