Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy
- PMID: 38254356
- PMCID: PMC10812546
- DOI: 10.3390/ani14020186
Ursolic Acid Induces Beneficial Changes in Skeletal Muscle mRNA Expression and Increases Exercise Participation and Performance in Dogs with Age-Related Muscle Atrophy
Abstract
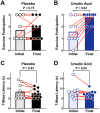
Muscle atrophy and weakness are prevalent and debilitating conditions in dogs that cannot be reliably prevented or treated by current approaches. In non-canine species, the natural dietary compound ursolic acid inhibits molecular mechanisms of muscle atrophy, leading to improvements in muscle health. To begin to translate ursolic acid to canine health, we developed a novel ursolic acid dietary supplement for dogs and confirmed its safety and tolerability in dogs. We then conducted a randomized, placebo-controlled, proof-of-concept efficacy study in older beagles with age-related muscle atrophy, also known as sarcopenia. Animals received placebo or ursolic acid dietary supplements once a day for 60 days. To assess the study's primary outcome, we biopsied the quadriceps muscle and quantified atrophy-associated mRNA expression. Additionally, to determine whether the molecular effects of ursolic acid might have functional correlates consistent with improvements in muscle health, we assessed secondary outcomes of exercise participation and T-maze performance. Importantly, in canine skeletal muscle, ursolic acid inhibited numerous mRNA expression changes that are known to promote muscle atrophy and weakness. Furthermore, ursolic acid significantly improved exercise participation and T-maze performance. These findings identify ursolic acid as a natural dietary compound that inhibits molecular mechanisms of muscle atrophy and improves functional performance in dogs.
Keywords: activity; cachexia; canine; dietary supplement; dog; exercise; muscle atrophy; sarcopenia; skeletal muscle; ursolic acid.
Conflict of interest statement
C.S.N., P.S., J.G.L., A.T.T. and C.A.R. are employees of Virbac; C.M.A., S.M.E., J.J.T., A.J.R. and B.B.R. are shareholders in Emmyon, Inc.; C.M.A., S.M.E. and J.J.T. serve as officers at Emmyon, Inc.; and B.B.R., A.R.J. and S.M.J. serve as consultants for Emmyon, Inc.
Figures


References
-
- Freeman L.M. Cachexia and Sarcopenia in Companion Animals: An under-Utilized Natural Animal Model of Human Disease. JCSM Rapid Commun. 2018;1:1–17. doi: 10.1002/j.2617-1619.2018.tb00006.x. - DOI
-
- Sacheck J.M., Hyatt J.-P.K., Raffaello A., Jagoe R.T., Roy R.R., Edgerton V.R., Lecker S.H., Goldberg A.L. Rapid Disuse and Denervation Atrophy Involve Transcriptional Changes Similar to Those of Muscle Wasting during Systemic Diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. - DOI - PubMed
Grants and funding
- R01AR060209/NH/NIH HHS/United States
- R01 AR081648/AR/NIAMS NIH HHS/United States
- R01 AG064386/AG/NIA NIH HHS/United States
- R01 AR060209/AR/NIAMS NIH HHS/United States
- R44 CA277853/CA/NCI NIH HHS/United States
- P30AG044271/NH/NIH HHS/United States
- P30 AG044271/AG/NIA NIH HHS/United States
- R01CA270025/NH/NIH HHS/United States
- R01AR081648/NH/NIH HHS/United States
- P30AG013319/NH/NIH HHS/United States
- R44CA277853/NH/NIH HHS/United States
- R01 AG060637/AG/NIA NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- R01AG060637/NH/NIH HHS/United States
- R01AG064386/NH/NIH HHS/United States
- R01 CA270025/CA/NCI NIH HHS/United States
- R44 AR069400/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

