Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse
- PMID: 38254668
- PMCID: PMC10812943
- DOI: 10.3390/biom14010068
Effect of Topical Programmed Death-Ligand1 on Corneal Epithelium in Dry Eye Mouse
Abstract
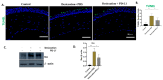
Dry eye disease (DED) is a growing health concern that impacts millions of individuals every year, and is associated with corneal injury, excessive oxidative stress and inflammation. Current therapeutic strategies, including artificial tears and anti-inflammatory agents, are unable to achieve a permanent clinical cure due to their temporary nature or adverse side effects. Therefore, here, we investigated the effectiveness of the topical administration of programmed death-ligand 1 (PD-L1) in the mouse model of DED. The model was generated in C57BL/6 mice by excising the extra orbital lacrimal gland and causing desiccation stress with scopolamine injections. Subsequently, either phosphate-buffered saline (3 µL/eye) or PD-L1 (0.5 µg/mL) was topically administered for 10 days. Tear volume was evaluated with phenol red thread, and corneal fluorescein staining was observed to quantify the corneal epithelial defect. Corneas were collected for histological analysis, and the expression levels of inflammatory signaling proteins such as CD4, CD3e, IL-17, IL-1β, pIkB-α, pNF-kB and pERK1/2 were assessed through immunofluorescence and Western blot techniques. Our results demonstrate that desiccating stress-induced corneal epithelial defect and tear secretion were significantly improved by topical PD-L1 and could reduce corneal CD4+ T cell infiltration, inflammation and apoptosis in a DED mouse model by downregulating IL-17 production and ERK1/2-NFkB pathways.
Keywords: corneal epithelial; desiccation stress; dry eye disease; programmed death-ligand 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

