Leveraging Biomaterial Platforms to Study Aging-Related Neural and Muscular Degeneration
- PMID: 38254669
- PMCID: PMC10813704
- DOI: 10.3390/biom14010069
Leveraging Biomaterial Platforms to Study Aging-Related Neural and Muscular Degeneration
Abstract
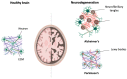
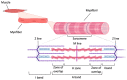
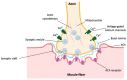
Aging is a complex multifactorial process that results in tissue function impairment across the whole organism. One of the common consequences of this process is the loss of muscle mass and the associated decline in muscle function, known as sarcopenia. Aging also presents with an increased risk of developing other pathological conditions such as neurodegeneration. Muscular and neuronal degeneration cause mobility issues and cognitive impairment, hence having a major impact on the quality of life of the older population. The development of novel therapies that can ameliorate the effects of aging is currently hindered by our limited knowledge of the underlying mechanisms and the use of models that fail to recapitulate the structure and composition of the cell microenvironment. The emergence of bioengineering techniques based on the use of biomimetic materials and biofabrication methods has opened the possibility of generating 3D models of muscular and nervous tissues that better mimic the native extracellular matrix. These platforms are particularly advantageous for drug testing and mechanistic studies. In this review, we discuss the developments made in the creation of 3D models of aging-related neuronal and muscular degeneration and we provide a perspective on the future directions for the field.
Keywords: 3D muscular models; 3D neural models; aging; biomaterials.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues.Acta Biomater. 2020 Jul 15;111:1-19. doi: 10.1016/j.actbio.2020.05.031. Epub 2020 May 25. Acta Biomater. 2020. PMID: 32464269 Review.
-
Converging functionality: Strategies for 3D hybrid-construct biofabrication and the role of composite biomaterials for skeletal regeneration.Acta Biomater. 2021 Sep 15;132:188-216. doi: 10.1016/j.actbio.2021.03.008. Epub 2021 Mar 10. Acta Biomater. 2021. PMID: 33713862 Review.
-
3D biomaterial models of human brain disease.Neurochem Int. 2021 Jul;147:105043. doi: 10.1016/j.neuint.2021.105043. Epub 2021 Apr 20. Neurochem Int. 2021. PMID: 33887378 Review.
-
Decellularized Extracellular Matrix for Bioengineering Physiomimetic 3D in Vitro Tumor Models.Trends Biotechnol. 2020 Dec;38(12):1397-1414. doi: 10.1016/j.tibtech.2020.04.006. Epub 2020 May 13. Trends Biotechnol. 2020. PMID: 32416940 Review.
-
Advancements in Extracellular Matrix-Based Biomaterials and Biofabrication of 3D Organotypic Skin Models.ACS Biomater Sci Eng. 2022 Aug 8;8(8):3220-3241. doi: 10.1021/acsbiomaterials.2c00342. Epub 2022 Jul 21. ACS Biomater Sci Eng. 2022. PMID: 35861577 Review.
Cited by
-
A review on innovations in hydroxyapatite: advancing sustainable and multifunctional dental implants.Odontology. 2025 Apr 10. doi: 10.1007/s10266-025-01096-3. Online ahead of print. Odontology. 2025. PMID: 40208376 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

