CSF-1R in Cancer: More than a Myeloid Cell Receptor
- PMID: 38254773
- PMCID: PMC10814415
- DOI: 10.3390/cancers16020282
CSF-1R in Cancer: More than a Myeloid Cell Receptor
Abstract
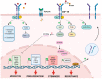
Colony-stimulating factor 1 receptor (CFS-1R) is a myeloid receptor with a crucial role in monocyte survival and differentiation. Its overexpression is associated with aggressive tumors characterized by an immunosuppressive microenvironment and poor prognosis. CSF-1R ligands, IL-34 and M-CSF, are produced by many cells in the tumor microenvironment (TME), suggesting a key role for the receptor in the crosstalk between tumor, immune and stromal cells in the TME. Recently, CSF-1R expression was reported in the cell membrane of the cancer cells of different solid tumors, capturing the interest of various research groups interested in investigating the role of this receptor in non-myeloid cells. This review summarizes the current data available on the expression and activity of CSF-1R in different tumor types. Notably, CSF-1R+ cancer cells have been shown to produce CSF-1R ligands, indicating that CSF-1R signaling is positively regulated in an autocrine manner in cancer cells. Recent research demonstrated that CSF-1R signaling enhances cell transformation by supporting tumor cell proliferation, invasion, stemness and drug resistance. In addition, this review covers recent therapeutic strategies, including monoclonal antibodies and small-molecule inhibitors, targeting the CSF-1R and designed to block the pro-oncogenic role of CSF-1R in cancer cells.
Keywords: CSF-1R; cancer cell signaling; cell migration; cell proliferation; chemoresistance; stemness.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

