Incidence of Cutaneous Immune-Related Adverse Events and Outcomes in Immune Checkpoint Inhibitor-Containing Regimens: A Systematic Review and Meta-Analysis
- PMID: 38254829
- PMCID: PMC10814132
- DOI: 10.3390/cancers16020340
Incidence of Cutaneous Immune-Related Adverse Events and Outcomes in Immune Checkpoint Inhibitor-Containing Regimens: A Systematic Review and Meta-Analysis
Abstract
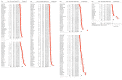
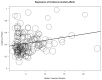
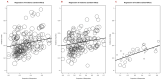
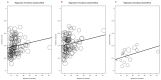
Immune checkpoint inhibitors (ICIs) are used to treat many cancers, and cutaneous immune-related adverse events (cirAEs) are among the most frequently encountered toxic effects. Understanding the incidence and prognostic associations of cirAEs is of importance as their uses in different settings, combinations, and tumor types expand. To evaluate the incidence of cirAEs and their association with outcome measures across a variety of ICI regimens and cancers, we performed a systematic review and meta-analysis of published trials of anti-programmed death-1/ligand-1 (PD-1/PD-L1) and anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) ICIs, both alone and in combination with chemotherapy, antiangiogenic agents, or other ICIs in patients with melanoma, renal cell carcinoma, non-small cell lung cancer, and urothelial carcinoma. Key findings of our study include variable cirAE incidence among tumors and ICI regimens, positive association with increased cirAE incidence and response rate, as well as significant association between increased vitiligo incidence and overall survival. Across 174 studies, rash, pruritis, and vitiligo were the most reported cirAEs, with incidences of 16.7%, 18.0%, and 6.6%, respectively. Higher incidence of cirAEs was associated with ICI combination regimens and with CTLA-4-containing regimens, particularly with higher doses of ipilimumab, as compared to PD-1/L1 monotherapies. Outcome measures including response rate and progression-free survival were positively correlated with incidence of cirAEs. The response rate and incidence of pruritis, vitiligo, and rash were associated with expected rises in incidence of 0.17% (p = 0.0238), 0.40% (p = 0.0010), and 0.18% (p = 0.0413), respectively. Overall survival was positively correlated with the incidence of pruritis, vitiligo, and rash; this association was significant for vitiligo (p = 0.0483). Our analysis provides benchmark incidence rates for cirAEs and links cirAEs with favorable treatment outcomes at a study level across diverse solid tumors and multiple ICI regimens.
Keywords: anti-angiogenic; autoimmune toxicities; chemotherapy; cutaneous immune-related adverse events; immune checkpoint inhibitors; melanoma; meta-analysis; non-small cell lung cancer; renal cell carcinoma; urothelial carcinoma.
Conflict of interest statement
D.B.J. has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, has received research funding from BMS and Incyte, and has patents pending for the use of MHC-II as a biomarker for immune checkpoint inhibitor response and abatacept as a treatment for immune-related adverse events.
Figures



References
-
- Cosio T., Coniglione F., Flaminio V., Gaziano R., Coletta D., Petruccelli R., Dika E., Bianchi L., Campione E. Pyodermitis during Nivolumab Treatment for Non-Small Cell Lung Cancer: A Case Report and Review of the Literature. Int. J. Mol. Sci. 2023;24:4580. doi: 10.3390/ijms24054580. - DOI - PMC - PubMed
-
- Zhang S., Tang K., Wan G., Nguyen N., Lu C., Ugwu-Dike P., Raval N., Seo J., Alexander N.A., Jairath R., et al. Cutaneous immune-related adverse events are associated with longer overall survival in advanced cancer patients on immune checkpoint inhibitors: A multi-institutional cohort study. J. Am. Acad. Dermatol. 2023;88:1024–1032. doi: 10.1016/j.jaad.2022.12.048. - DOI - PMC - PubMed
-
- Tang K., Seo J., Tiu B.C., Le T.K., Pahalyants V., Raval N.S., Ugwu-Dike P.O., Zubiri L., Naranbhai V., Carrington M., et al. Association of Cutaneous Immune-Related Adverse Events With Increased Survival in Patients Treated With Anti–Programmed Cell Death 1 and Anti–Programmed Cell Death Ligand 1 Therapy. JAMA Dermatol. 2022;158:189–193. doi: 10.1001/jamadermatol.2021.5476. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

