Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma
- PMID: 38254857
- PMCID: PMC10814383
- DOI: 10.3390/cancers16020368
Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma
Abstract
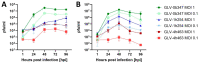
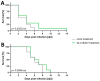
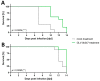
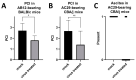
Effective treatment options for peritoneal surface malignancies (PSMs) are scarce. Oncolytic virotherapy with recombinant vaccinia viruses might constitute a novel treatment option for PSM. We aimed to identify the most effective oncolytic vaccinia virus strain in two murine mesothelioma cell lines and the oncolytic potential in a murine model of peritoneal mesothelioma. Cell lines AB12 and AC29 were infected in vitro with vaccinia virus strains Lister (GLV-1h254), Western Reserve (GLV-0b347), and Copenhagen (GLV-4h463). The virus strain GLV-0b347 was shown most effective in vitro and was further investigated by intraperitoneal (i.p.) application to AB12 and AC29 mesothelioma-bearing mice. Feasibility, safety, and effectiveness of virotherapy were assessed by evaluating the peritoneal cancer index (PCI), virus detection in tumor tissues and ascites, virus growth curves, and comparison of overall survival. After i.p. injection of GLV-0b347, virus was detected in both tumor cells and ascites. In comparison to mock-treated mice, overall survival was significantly prolonged, ascites was less frequent and PCI values declined. However, effective treatment was only observed in animals with limited tumor burden at the time point of virus application. Nonetheless, intraperitoneal virotherapy with GLV-0b347 might constitute a novel therapeutic option for the treatment of peritoneal mesothelioma. Additional treatment modifications and combinational regimes will be investigated to further enhance treatment efficacy.
Keywords: immunovirotherapy; intraperitoneal therapy; oncolytic virotherapy; peritoneal carcinomatosis; syngeneic murine tumor model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Gene therapy using therapeutic and diagnostic recombinant oncolytic vaccinia virus GLV-1h153 for management of colorectal peritoneal carcinomatosis.Surgery. 2015 Feb;157(2):331-7. doi: 10.1016/j.surg.2014.09.008. Surgery. 2015. PMID: 25616946 Free PMC article.
-
Oncolytic vaccinia virus as an adjuvant treatment to cytoreductive surgery for malignant peritoneal mesothelioma.Ann Surg Oncol. 2014 Jul;21(7):2259-66. doi: 10.1245/s10434-014-3651-4. Epub 2014 Apr 10. Ann Surg Oncol. 2014. PMID: 24719018
-
Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model.Viruses. 2023 Jan 27;15(2):363. doi: 10.3390/v15020363. Viruses. 2023. PMID: 36851574 Free PMC article.
-
Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68.J Transl Med. 2013 Mar 26;11:79. doi: 10.1186/1479-5876-11-79. J Transl Med. 2013. PMID: 23531320 Free PMC article.
-
Oncolytic Viral Therapy for Mesothelioma.Front Oncol. 2017 Aug 24;7:179. doi: 10.3389/fonc.2017.00179. eCollection 2017. Front Oncol. 2017. PMID: 28884088 Free PMC article. Review.
References
-
- Yan T.D., Deraco M., Baratti D., Kusamura S., Elias D., Glehen O., Gilly F.N., Levine E.A., Shen P., Mohamed F., et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J. Clin. Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. - DOI - PubMed
-
- Gilani S.N.S., Mehta A., Garcia-Fadrique A., Rowaiye B., Jenei V., Dayal S., Chandrakumaran K., Carr N., Mohamed F., Cecil T., et al. Outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma and predictors of survival. Int. J. Hyperthermia. 2018;34:578–584. doi: 10.1080/02656736.2018.1434902. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

