Combinational Pulsing of TAAs Enforces Dendritic Cell-Based Immunotherapy through T-Cell Proliferation and Interferon-γ Secretion in LLC1 Mouse Model
- PMID: 38254898
- PMCID: PMC10814594
- DOI: 10.3390/cancers16020409
Combinational Pulsing of TAAs Enforces Dendritic Cell-Based Immunotherapy through T-Cell Proliferation and Interferon-γ Secretion in LLC1 Mouse Model
Abstract
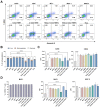
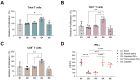
NSCLC, the most common type of lung cancer, is often diagnosed late due to minimal early symptoms. Its high risk of recurrence or metastasis post-chemotherapy makes DC-based immunotherapy a promising strategy, offering targeted cancer destruction, low side effects, memory formation, and overcoming the immune evasive ability of cancers. However, the limited response to DCs pulsed with single antigens remains a significant challenge. To overcome this, we enhanced DC antigen presentation by pulsing with TAAs. Our study focused on enhancing DC-mediated immune response specificity and intensity by combinatorial pulsing of TAAs, selected for their prevalence in NSCLC. We selected four types of TAAs expressed in NSCLC and pulsed DCs with the optimal combination. Next, we administered TAAs-pulsed DCs into the LLC1 mouse model to evaluate their anti-tumor efficacy. Our results showed that TAAs-pulsed DCs significantly reduced tumor size and promoted apoptosis in tumor tissue. Moreover, TAAs-pulsed DCs significantly increased total T cells in the spleen compared to the unpulsed DCs. Additionally, in vitro stimulation of splenocytes from the TAAs-pulsed DCs showed notable T-cell proliferation and increased IFN-γ secretion. Our findings demonstrate the potential of multiple TAA pulsing to enhance the antigen-presenting capacity of DCs, thereby strengthening the immune response against tumors.
Keywords: dendritic cells; immunotherapy; non-small cell lung cancer; tumor-associated antigen.
Conflict of interest statement
The authors affiliated with Affiliation 1 and Affiliation 2 are employees of EHLBio Co., Ltd. and EHLCell Clinic, with Hong-Ki Lee serving as the CEO of both companies. They declare no competing financial interests, such as holding stocks or any other financial involvement related to the content of this paper. This study was conducted with complete scientific integrity and independence.
Figures



References
-
- Pignon J.P., Tribodet H., Scagliotti G.V., Douillard J.Y., Shepherd F.A., Stephens R.J., Dunant A., Torri V., Rosell R., Seymour L., et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. - DOI - PubMed
-
- Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. - DOI - PubMed
LinkOut - more resources
Full Text Sources

