Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review
- PMID: 38254900
- PMCID: PMC10814983
- DOI: 10.3390/cancers16020411
Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review
Abstract
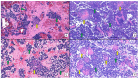
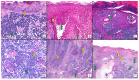
Combined Merkel cell carcinoma (MCC) and squamous cell carcinoma (SCC) have classically been regarded as more aggressive than conventional, pure, Merkel cell polyomavirus (MCPyV)-positive MCC. It is still unknown whether combined MCC and SCC are more aggressive than pure, MCPyV-negative MCC, and the origin of both the SCC and MCC elements of these combined tumors has not been elucidated. The main objective of this systematic review was to assess whether combined MCC and SCC tumors are associated with a worse prognosis than pure MCC; the secondary goals were the characterization of the clinical and histopathological features of these combined neoplasms. A total of 38 studies, including 152 patients, were selected for review. In total, 76% of the cases were MCPyV-negative, whereas 4% were MCPyV-positive. The most frequent histopathological pattern was that of an SCC in situ combined with a dermal MCC (36%), followed by both an in situ and invasive SCC combined with a dermal MCC (20%). Forty-seven percent of all cases fitted in the morphology of the so-called "collision tumors". Three combined MCC cases that would fit in the morphological category of collision tumors presented both squamous and neuroendocrine elements in their respective nodal metastases. The mean overall survival was 36 months, comparable to that of pure, MCPyV-negative MCC. This review found similarly aggressive behavior for combined MCC and SCC and pure, MCPyV-negative MCC. Preliminary data strongly suggest that all MCPyV-negative MCC tumors, whether combined or pure, are part of a common spectrum.
Keywords: Merkel cell carcinoma; Merkel cell polyomavirus; combined Merkel cell carcinoma; combined squamous cell carcinoma and Merkel cell carcinoma; divergent Merkel cell carcinoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Kervarrec T., Appenzeller S., Samimi M., Sarma B., Sarosi E.-M., Berthon P., Le Corre Y., Hainaut-Wierzbicka E., Blom A., Benethon N., et al. Merkel Cell Polyomavirus–Negative Merkel Cell Carcinoma Originating from In Situ Squamous Cell Carcinoma: A Keratinocytic Tumor with Neuroendocrine Differentiation. J. Investig. Dermatol. 2021;142:516–527. doi: 10.1016/j.jid.2021.07.175. - DOI - PubMed
-
- Kervarrec T., Tallet A., Miquelestorena-Standley E., Houben R., Schrama D., Gambichler T., Berthon P., Le Corre Y., Hainaut-Wierzbicka E., Aubin F., et al. Morphologic and Immunophenotypical Features Distinguishing Merkel Cell Polyomavirus-Positive and Negative Merkel Cell Carcinoma. Mod. Pathol. 2019;32:1605–1616. doi: 10.1038/s41379-019-0288-7. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

