Hypoxia Dysregulates the Transcription of Myoendothelial Junction Proteins Involved with Nitric Oxide Production in Brain Endothelial Cells
- PMID: 38255181
- PMCID: PMC10813549
- DOI: 10.3390/biomedicines12010075
Hypoxia Dysregulates the Transcription of Myoendothelial Junction Proteins Involved with Nitric Oxide Production in Brain Endothelial Cells
Abstract
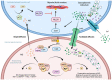
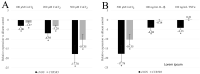
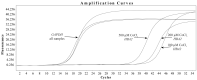
Myoendothelial junctions (MEJs) are structures that allow chemical signals to be transmitted between endothelial cells (ECs) and vascular smooth muscle cells, which control vascular tone. MEJs contain hemoglobin alpha (Hbα) and endothelial nitric oxide synthase (eNOS) complexes that appear to control the production and scavenging of nitric oxide (NO) along with the activity of cytochrome b5 reductase 3 (CYB5R3). The aim of this study was to examine how hypoxia affected the regulation of proteins involved in the production of NO in brain ECs. In brief, human brain microvascular endothelial cells (HBMEC) were exposed to cobalt chloride (CoCl2), a hypoxia mimetic, and a transcriptional analysis was performed using primers for eNOS, CYB5R3, and Hbα2 with ΔΔCt relative gene expression normalized to GAPDH. NO production was also measured after treatment using 4,5-diaminofluorescein diacetate (DAF-DA), a fluorescent NO indicator. When HBMEC were exposed to CoCl2 for 48 h, eNOS and CYB5R3 messenger RNA significantly decreased (up to -17.8 ± 4.30-fold and -10.4 ± 2.8, respectively) while Hbα2 increased to detectable levels. Furthermore, CoCl2 treatment caused a redistribution of peripheral membrane-generated NO production to a perinuclear region. To the best of our knowledge, this is the first time this axis has been studied in brain ECs and these findings imply that hypoxia may cause dysregulation of proteins that regulate NO production in brain MEJs.
Keywords: cytochrome b5 reductase 3 myoendothelial junction; endothelial nitric oxide synthase; hemoglobin alpha; nitric oxide.
Conflict of interest statement
The authors declare they have no conflicts of interest to report and that the views, opinions, findings, and conclusions or recommendations expressed in these papers and articles are strictly those of the author(s). They do not necessarily reflect the views of HCA Healthcare or South Texas Health System, its affiliates, or its parent company. HCA Healthcare and South Texas Health System take no responsibility for any errors or omissions in, or for the correctness of, the information contained in papers and articles.
Figures

Similar articles
-
Can endothelial hemoglobin-α regulate nitric oxide vasodilatory signaling?Am J Physiol Heart Circ Physiol. 2017 Apr 1;312(4):H854-H866. doi: 10.1152/ajpheart.00315.2016. Epub 2017 Jan 27. Am J Physiol Heart Circ Physiol. 2017. PMID: 28130333 Free PMC article.
-
Hemoglobin α/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction.Arterioscler Thromb Vasc Biol. 2014 Dec;34(12):2594-600. doi: 10.1161/ATVBAHA.114.303974. Epub 2014 Oct 2. Arterioscler Thromb Vasc Biol. 2014. PMID: 25278292 Free PMC article.
-
Endothelial cell regulation of nitric oxide production during hypoxia in coronary microvessels and epicardial arteries.J Cell Physiol. 2000 Mar;182(3):359-65. doi: 10.1002/(SICI)1097-4652(200003)182:3<359::AID-JCP6>3.0.CO;2-3. J Cell Physiol. 2000. PMID: 10653602
-
Hemoglobin α in the blood vessel wall.Free Radic Biol Med. 2014 Aug;73:136-42. doi: 10.1016/j.freeradbiomed.2014.04.019. Epub 2014 May 14. Free Radic Biol Med. 2014. PMID: 24832680 Free PMC article. Review.
-
Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel.Arterioscler Thromb Vasc Biol. 2004 Mar;24(3):405-12. doi: 10.1161/01.ATV.0000109171.50229.33. Epub 2003 Dec 1. Arterioscler Thromb Vasc Biol. 2004. PMID: 14656742 Review.
Cited by
-
The diagnostic and prognostic significance of methylated arginine metabolites (ADMA, SDMA, L-NMMA) in patients with obstructive sleep apnea syndrome.Medicine (Baltimore). 2025 Aug 8;104(32):e43903. doi: 10.1097/MD.0000000000043903. Medicine (Baltimore). 2025. PMID: 40797410 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

