Bielectrode Strategy for Determination of CYP2E1 Catalytic Activity: Electrodes with Bactosomes and Voltammetric Determination of 6-Hydroxychlorzoxazone
- PMID: 38255257
- PMCID: PMC10812958
- DOI: 10.3390/biomedicines12010152
Bielectrode Strategy for Determination of CYP2E1 Catalytic Activity: Electrodes with Bactosomes and Voltammetric Determination of 6-Hydroxychlorzoxazone
Abstract
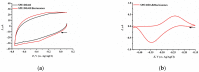
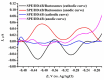
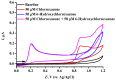
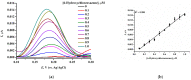
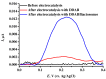
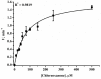
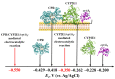
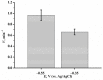
We describe a bielectrode system for evaluation of the electrocatalytic activity of cytochrome P450 2E1 (CYP2E1) towards chlorzoxazone. One electrode of the system was employed to immobilize Bactosomes with human CYP2E1, cytochrome P450 reductase (CPR), and cytochrome b5 (cyt b5). The second electrode was used to quantify CYP2E1-produced 6-hydroxychlorzoxazone by its direct electrochemical oxidation, registered using square-wave voltammetry. Using this system, we determined the steady-state kinetic parameters of chlorzoxazone hydroxylation by CYP2E1 of Bactosomes immobilized on the electrode: the maximal reaction rate (Vmax) was 1.64 ± 0.08 min-1, and the Michaelis constant (KM) was 78 ± 9 μM. We studied the electrochemical characteristics of immobilized Bactosomes and have revealed that electron transfer from the electrode occurs both to the flavin prosthetic groups of CPR and the heme iron ions of CYP2E1 and cyt b5. Additionally, it has been demonstrated that CPR has the capacity to activate CYP2E1 electrocatalytic activity towards chlorzoxazone, likely through intermolecular electron transfer from the electrochemically reduced form of CPR to the CYP2E1 heme iron ion.
Keywords: 6-hydroxychlorzoxazone; Bactosomes; CYP2E1; chlorzoxazone; screen-printed electrodes; voltammetry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Requirements for cytochrome b5 in the oxidation of 7-ethoxycoumarin, chlorzoxazone, aniline, and N-nitrosodimethylamine by recombinant cytochrome P450 2E1 and by human liver microsomes.Biochem Pharmacol. 1996 Jul 26;52(2):301-9. doi: 10.1016/0006-2952(96)00208-0. Biochem Pharmacol. 1996. PMID: 8694855
-
Development of a non-high pressure liquid chromatography assay to determine [14C]chlorzoxazone 6-hydroxylase (CYP2E1) activity in human liver microsomes.Drug Metab Dispos. 1998 Apr;26(4):305-12. Drug Metab Dispos. 1998. PMID: 9531516
-
Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans.Br J Clin Pharmacol. 2003 Jan;55(1):77-85. doi: 10.1046/j.1365-2125.2003.01731.x. Br J Clin Pharmacol. 2003. PMID: 12534643 Free PMC article.
-
Comprehensive kinetic analysis and influence of reaction components for chlorzoxazone 6-hydroxylation in human liver microsomes with CYP antibodies.Xenobiotica. 2015 Apr;45(4):353-60. doi: 10.3109/00498254.2014.985760. Epub 2014 Nov 19. Xenobiotica. 2015. PMID: 25815637
-
Chlorzoxazone is metabolized by human CYP1A2 as well as by human CYP2E1.Pharmacogenetics. 1995 Jun;5(3):143-50. doi: 10.1097/00008571-199506000-00002. Pharmacogenetics. 1995. PMID: 7550365
Cited by
-
Engineering Electron Transfer Pathway of Cytochrome P450s.Molecules. 2024 May 24;29(11):2480. doi: 10.3390/molecules29112480. Molecules. 2024. PMID: 38893355 Free PMC article. Review.
References
-
- Iacopetta D., Ceramella J., Catalano A., Scali E., Scumaci D., Pellegrino M., Aquaro S., Saturnino C., Sinicropi M.S. Impact of cytochrome P450 enzymes on the phase I metabolism of drugs. Appl. Sci. 2023;13:6045. doi: 10.3390/app13106045. - DOI
-
- Li H., Sheng Y., Li W., Yuan L. Recent advances in molecular fluorescent probes for CYP450 sensing and imaging. Chemosensors. 2022;10:304. doi: 10.3390/chemosensors10080304. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

