Co-Administration of Proton Pump Inhibitors May Negatively Affect the Outcome in Inflammatory Bowel Disease Treated with Vedolizumab
- PMID: 38255263
- PMCID: PMC10813460
- DOI: 10.3390/biomedicines12010158
Co-Administration of Proton Pump Inhibitors May Negatively Affect the Outcome in Inflammatory Bowel Disease Treated with Vedolizumab
Abstract
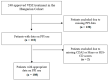
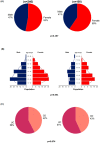
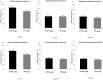
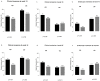
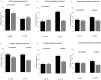
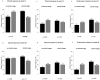
Concomitant medications may alter the effect of biological therapy in inflammatory bowel disease. The aim was to investigate the effect of proton pump inhibitors on remission rates in patients with inflammatory bowel disease treated with the gut-selective vedolizumab. Patients from the Hungarian nationwide, multicenter vedolizumab cohort were selected for post hoc analysis. Primary outcomes were the assessment of clinical response and endoscopic and clinical remission at weeks 14 and 54. Secondary outcomes were the evaluation of the combined effect of concomitant steroid therapy and other factors, such as smoking, on remission. A total of 108 patients were identified with proton pump inhibitor data from 240 patients in the original cohort. Patients on steroids without proton pump inhibitors were more likely to have a clinical response at week 14 than patients on concomitant PPI (95% vs. 67%, p = 0.005). Non-smokers with IBD treated with VDZ were more likely to develop a clinical response at week 14 than smokers, particularly those not receiving PPI compared with patients on co-administered PPI therapy (81% vs. 53%, p = 0.041, and 92% vs. 74%, p = 0.029, respectively). We found that the use of PPIs in patients treated with VDZ may impair the achievement of response in certain subgroups. Unnecessary PPI prescriptions should be avoided.
Keywords: inflammatory bowel disease; proton pump inhibitor; vedolizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
- FK 132834/National Research, Development and Innovation Office
- ÚNKP-22-5-PTE-1737/ÚNKP-22-5 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund
- BO/00317/21/János Bolyai Research Scholarship of the Hungarian Academy of Sciences
LinkOut - more resources
Full Text Sources

