Menopause-Associated Depression: Impact of Oxidative Stress and Neuroinflammation on the Central Nervous System-A Review
- PMID: 38255289
- PMCID: PMC10813042
- DOI: 10.3390/biomedicines12010184
Menopause-Associated Depression: Impact of Oxidative Stress and Neuroinflammation on the Central Nervous System-A Review
Abstract
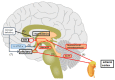
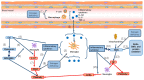
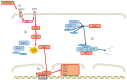
Perimenopausal depression, occurring shortly before or after menopause, is characterized by symptoms such as emotional depression, anxiety, and stress, often accompanied by endocrine dysfunction, particularly hypogonadism and senescence. Current treatments for perimenopausal depression primarily provide symptomatic relief but often come with undesirable side effects. The development of agents targeting the specific pathologies of perimenopausal depression has been relatively slow. The erratic fluctuations in estrogen and progesterone levels during the perimenopausal stage expose women to the risk of developing perimenopausal-associated depression. These hormonal changes trigger the production of proinflammatory mediators and induce oxidative stress, leading to progressive neuronal damage. This review serves as a comprehensive overview of the underlying mechanisms contributing to perimenopausal depression. It aims to shed light on the complex relationship between perimenopausal hormones, neurotransmitters, brain-derived neurotrophic factors, chronic inflammation, oxidative stress, and perimenopausal depression. By summarizing the intricate interplay between hormonal fluctuations, neurotransmitter activity, brain-derived neurotrophic factors, chronic inflammation, oxidative stress, and perimenopausal depression, this review aims to stimulate further research in this field. The hope is that an increased understanding of these mechanisms will pave the way for the development of more effective therapeutic targets, ultimately reducing the risk of depression during the menopausal stage for the betterment of psychological wellbeing.
Keywords: estrogen deprivation; pro-inflammatory cytokines; psychological wellbeing.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the writing of the manuscript.
Figures
References
-
- Gorlova A., Svirin E., Pavlov D., Cespuglio R., Proshin A., Schroeter C.A., Lesch K.P., Strekalova T. Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. Int. J. Mol. Sci. 2023;24:915. doi: 10.3390/ijms24020915. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

