Aptamer-Based Smart Targeting and Spatial Trigger-Response Drug-Delivery Systems for Anticancer Therapy
- PMID: 38255292
- PMCID: PMC10813750
- DOI: 10.3390/biomedicines12010187
Aptamer-Based Smart Targeting and Spatial Trigger-Response Drug-Delivery Systems for Anticancer Therapy
Abstract
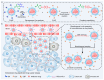
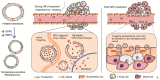
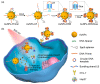
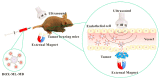
In recent years, the field of drug delivery has witnessed remarkable progress, driven by the quest for more effective and precise therapeutic interventions. Among the myriad strategies employed, the integration of aptamers as targeting moieties and stimuli-responsive systems has emerged as a promising avenue, particularly in the context of anticancer therapy. This review explores cutting-edge advancements in targeted drug-delivery systems, focusing on the integration of aptamers and stimuli-responsive platforms for enhanced spatial anticancer therapy. In the aptamer-based drug-delivery systems, we delve into the versatile applications of aptamers, examining their conjugation with gold, silica, and carbon materials. The synergistic interplay between aptamers and these materials is discussed, emphasizing their potential in achieving precise and targeted drug delivery. Additionally, we explore stimuli-responsive drug-delivery systems with an emphasis on spatial anticancer therapy. Tumor microenvironment-responsive nanoparticles are elucidated, and their capacity to exploit the dynamic conditions within cancerous tissues for controlled drug release is detailed. External stimuli-responsive strategies, including ultrasound-mediated, photo-responsive, and magnetic-guided drug-delivery systems, are examined for their role in achieving synergistic anticancer effects. This review integrates diverse approaches in the quest for precision medicine, showcasing the potential of aptamers and stimuli-responsive systems to revolutionize drug-delivery strategies for enhanced anticancer therapy.
Keywords: aptamer; stimuli-responsive drug delivery; targeted drug delivery.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures












References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

