The Traumatic Inoculation Process Affects TSPO Radioligand Uptake in Experimental Orthotopic Glioblastoma
- PMID: 38255293
- PMCID: PMC10813339
- DOI: 10.3390/biomedicines12010188
The Traumatic Inoculation Process Affects TSPO Radioligand Uptake in Experimental Orthotopic Glioblastoma
Abstract
Background: The translocator protein (TSPO) has been proven to have great potential as a target for the positron emission tomography (PET) imaging of glioblastoma. However, there is an ongoing debate about the potential various sources of the TSPO PET signal. This work investigates the impact of the inoculation-driven immune response on the PET signal in experimental orthotopic glioblastoma.
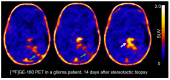
Methods: Serial [18F]GE-180 and O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) PET scans were performed at day 7/8 and day 14/15 after the inoculation of GL261 mouse glioblastoma cells (n = 24) or saline (sham, n = 6) into the right striatum of immunocompetent C57BL/6 mice. An additional n = 25 sham mice underwent [18F]GE-180 PET and/or autoradiography (ARG) at days 7, 14, 21, 28, 35, 50 and 90 in order to monitor potential reactive processes that were solely related to the inoculation procedure. In vivo imaging results were directly compared to tissue-based analyses including ARG and immunohistochemistry.
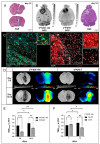
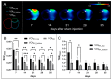
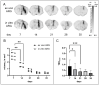
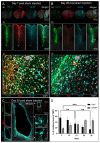
Results: We found that the inoculation process represents an immunogenic event, which significantly contributes to TSPO radioligand uptake. [18F]GE-180 uptake in GL261-bearing mice surpassed [18F]FET uptake both in the extent and the intensity, e.g., mean target-to-background ratio (TBRmean) in PET at day 7/8: 1.22 for [18F]GE-180 vs. 1.04 for [18F]FET, p < 0.001. Sham mice showed increased [18F]GE-180 uptake at the inoculation channel, which, however, continuously decreased over time (e.g., TBRmean in PET: 1.20 at day 7 vs. 1.09 at day 35, p = 0.04). At the inoculation channel, the percentage of TSPO/IBA1 co-staining decreased, whereas TSPO/GFAP (glial fibrillary acidic protein) co-staining increased over time (p < 0.001).
Conclusion: We identify the inoculation-driven immune response to be a relevant contributor to the PET signal and add a new aspect to consider for planning PET imaging studies in orthotopic glioblastoma models.
Keywords: TSPO PET and autoradiography; glioblastoma; immunohistochemistry; orthotopic implantation; traumatic brain injury (TBI).
Conflict of interest statement
The authors declare no competing interests. M.B. received speaker honoraria from GE healthcare, Roche and LMI and is an advisor of LMI and MIAC. N.L.A. has received honoraria for consultation or advisory board participation from Novartis/Advanced Accelerator Applications, Telix Pharmaceuticals and Servier and research funding from Novocure. A.H. is an advisor of ABX advanced biochemical compounds.
Figures
References
-
- Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021;18:170–186. doi: 10.1038/s41571-020-00447-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

