Enhanced Enzymatic Synthesis of Puerarin Palmitate with Different Acyl Donors for Lipid Solubility Improvement
- PMID: 38255784
- PMCID: PMC10815456
- DOI: 10.3390/ijms25020709
Enhanced Enzymatic Synthesis of Puerarin Palmitate with Different Acyl Donors for Lipid Solubility Improvement
Abstract
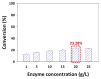
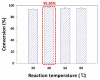
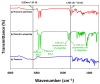
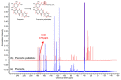
Puerarin is a flavonoid known as a natural antioxidant found in the root of Pueraria robata. Its antioxidant, anticancer, and anti-inflammatory effects have attracted attention as a potential functional ingredient in various bioindustries. However, puerarin has limited bioavailability owing to its low lipid solubility and stability. Acylation is proposed as a synthesis method to overcome this limitation. In this study, lipase-catalyzed acylation of puerarin and various acyl donors was performed, and the enzymatic synthetic condition was optimized. Under the condition (20 g/L of Novozym 435, palmitic anhydride, 1:15, 40 °C, tetrahydrofuran (THF)), the synthesis of puerarin ester achieved a significantly high conversion (98.97%) within a short time (3 h). The molecule of the synthesized puerarin palmitate was identified by various analyses such as liquid chromatography-mass spectrometry (LC-MS), Fourier-transform infrared spectroscopy (FT-IR), and carbon-13 nuclear magnetic resonance (13C NMR). The lipid solubility and the radical scavenging activity were also evaluated. Puerarin palmitate showed a slight decrease in antioxidant activity, but lipid solubility was significantly improved, improving bioavailability. The high conversion achieved for puerarin esters in this study will provide the foundation for industrial applications.
Keywords: antioxidant; flavonoid; flavonoid ester; lipase; lipid solubility; puerarin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

