Mechanism of Iron Ion Homeostasis in Intestinal Immunity and Gut Microbiota Remodeling
- PMID: 38255801
- PMCID: PMC10815743
- DOI: 10.3390/ijms25020727
Mechanism of Iron Ion Homeostasis in Intestinal Immunity and Gut Microbiota Remodeling
Abstract
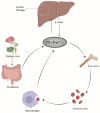
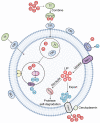
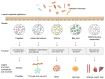
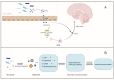
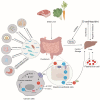
Iron is a vital trace element that plays an important role in humans and other organisms. It plays an active role in the growth, development, and reproduction of bacteria, such as Bifidobacteria. Iron deficiency or excess can negatively affect bacterial hosts. Studies have reported a major role of iron in the human intestine, which is necessary for maintaining body homeostasis and intestinal barrier function. Organisms can maintain their normal activities and regulate some cancer cells in the body by regulating iron excretion and iron-dependent ferroptosis. In addition, iron can modify the interaction between hosts and microorganisms by altering their growth and virulence or by affecting the immune system of the host. Lactic acid bacteria such as Lactobacillus acidophilus (L. acidophilus), Lactobacillus rhamnosus (L. rhamnosus), and Lactobacillus casei (L. casei) were reported to increase trace elements, protect the host intestinal barrier, mitigate intestinal inflammation, and regulate immune function. This review article focuses on the two aspects of the iron and gut and generally summarizes the mechanistic role of iron ions in intestinal immunity and the remodeling of gut microbiota.
Keywords: gut; gut microbiota; intestinal immunity; iron; iron homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Li H., Yang T., Liao T., Debowski A.W., Nilsson H.-O., Haslam S.M., Dell A., Stubbs K.A., Marshall B.J., Benghezal M. Insights from the redefinition of Helicobacter pylori lipopolysaccharide O-antigen and core-oligosaccharide domains. Microb. Cell. 2017;4:175–178. doi: 10.15698/mic2017.05.574. - DOI - PMC - PubMed
-
- Maes M., Vojdani A., Sirivichayakul S., Barbosa D.S., Kanchanatawan B. Inflammatory and Oxidative Pathways Are New Drug Targets in Multiple Episode Schizophrenia and Leaky Gut, Klebsiella pneumoniae, and C1q Immune Complexes Are Additional Drug Targets in First Episode Schizophrenia. Mol. Neurobiol. 2021;58:3319–3334. doi: 10.1007/s12035-021-02343-8. - DOI - PubMed
-
- Overacre-Delgoffe A.E., Bumgarner H.J., Cillo A.R., Burr A.H.P., Tometich J.T., Bhattacharjee A., Bruno T.C., Vignali D.A.A., Hand T.W. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. 2021;54:2812–2824.e4. doi: 10.1016/j.immuni.2021.11.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

