Evaluation of the LDN-0060609 PERK Inhibitor as a Selective Treatment for Primary Open-Angle Glaucoma: An In Vitro Study on Human Retinal Astrocytes
- PMID: 38255802
- PMCID: PMC10815359
- DOI: 10.3390/ijms25020728
Evaluation of the LDN-0060609 PERK Inhibitor as a Selective Treatment for Primary Open-Angle Glaucoma: An In Vitro Study on Human Retinal Astrocytes
Abstract
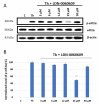
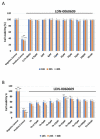
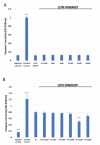
The term glaucoma encompasses various neurodegenerative eye disorders, among which the most common is primary open-angle glaucoma (POAG). Recently, the essential role of human retinal astrocytes (HRA) in glaucoma progression has been placed in the spotlight. It has been found that placing the endoplasmic reticulum (ER) under stress and activating PERK leads to apoptosis of HRA cells, which inhibits their neuroprotective effect in the course of glaucoma. Therefore, the aim of the present study was to evaluate the effectiveness of the small-molecule PERK inhibitor LDN-0060609 in countering ER stress conditions induced in HRA cells in vitro. The activity of LDN-0060609 was studied in terms of protein and mRNA expression, cytotoxicity, genotoxicity, caspase-3 level and cell cycle progression. LDN-0060609 at 25 μM proved to be a potent inhibitor of the major PERK substrate, p-eIF2α (49% inhibition). The compound markedly decreased the expression of pro-apoptotic ER stress-related genes (ATF4, DDIT3, BAX and Bcl-2). Treatment with LDN-0060609 significantly increased cell viability, decreased genotoxicity and caspase-3 levels, and restored cell cycle distribution in HRA cells with activated ER stress conditions. These findings indicate that the small-molecule PERK inhibitor LDN-0060609 can potentially be developed into a novel anti-glaucoma agent.
Keywords: PERK; PERK inhibitor; eIF2α; endoplasmic reticulum stress; glaucoma; glaucoma treatment; unfolded protein response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Burton M.J., Ramke J., Marques A.P., Bourne R.R.A., Congdon N., Jones I., Ah Tong B.A.M., Arunga S., Bachani D., Bascaran C., et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health. 2021;9:e489–e551. doi: 10.1016/S2214-109X(20)30488-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

