The Use of Nitrosative Stress Molecules as Potential Diagnostic Biomarkers in Multiple Sclerosis
- PMID: 38255863
- PMCID: PMC10815836
- DOI: 10.3390/ijms25020787
The Use of Nitrosative Stress Molecules as Potential Diagnostic Biomarkers in Multiple Sclerosis
Abstract
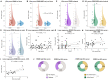
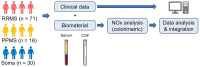
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) of still unclear etiology. In recent years, the search for biomarkers facilitating its diagnosis, prognosis, therapy response, and other parameters has gained increasing attention. In this regard, in a previous meta-analysis comprising 22 studies, we found that MS is associated with higher nitrite/nitrate (NOx) levels in the cerebrospinal fluid (CSF) compared to patients with non-inflammatory other neurological diseases (NIOND). However, many of the included studies did not distinguish between the different clinical subtypes of MS, included pre-treated patients, and inclusion criteria varied. As a follow-up to our meta-analysis, we therefore aimed to analyze the serum and CSF NOx levels in clinically well-defined cohorts of treatment-naïve MS patients compared to patients with somatic symptom disorder. To this end, we analyzed the serum and CSF levels of NOx in 117 patients (71 relapsing-remitting (RR) MS, 16 primary progressive (PP) MS, and 30 somatic symptom disorder). We found that RRMS and PPMS patients had higher serum NOx levels compared to somatic symptom disorder patients. This difference remained significant in the subgroup of MRZ-negative RRMS patients. In conclusion, the measurement of NOx in the serum might indeed be a valuable tool in supporting MS diagnosis.
Keywords: biomarkers; nitrosative stress molecules; primary progressive multiple sclerosis; relapsing remitting multiple sclerosis.
Conflict of interest statement
S.R. received travel grants from Merck Healthcare Germany GmbH, Alexion Pharmaceuticals, Bristol Myers Squibb, the German Neurological Society, and the American Academy of Neurology. She served on a scientific advisory board from Merck Healthcare Germany GmbH and received honoraria for lecturing from Roche. Her research was supported by Novartis, ‘Stiftung zur Förderung junger Neurowissenschaftler’ and ‘Else Kröner-Fresenius-Stiftung’. M.F. received travel grants from Merck Healthcare Germany GmbH, Novartis, the German Neurological Society, and benefitted in kind from Biogen. He served on a scientific advisory board from Swedish Orphan Biovitrum GmbH. C.B.S. received travel grants from Alexion Pharmaceuticals. She served on a scientific advisory board from Merck Healthcare Germany GmbH. N.H. received honoraria for lecturing meetings from Argenx and travel reimbursements and meeting attendance fees from Alexion, Merck, and Novartis. He served on a scientific advisory board from Viatris. M.P. received honoraria for lecturing and travel expenses for attending meetings from Alexion, ArgenX, Bayer Health Care, Biogen, Hexal, Merck Serono, Novartis, Roche, Sanofi-Aventis, and Teva. His research is funded by Biogen, Hexal, Merck Serono, Novartis, and Teva. S.G.M. received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer HealthCare, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Bundesinstitut für Risikobewertung (BfR), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Gemeinsamer Bundesausschuss (G-BA), German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Alexion, Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. N.M. has received honoraria for lecturing and travel expenses for attending meetings from Biogen Idec, GlaxoSmith Kline, Teva, Novartis Pharma, Bayer Healthcare, Genzyme, Alexion Pharmaceuticals, Fresenius Medical Care, Diamed, UCB Pharma, AngeliniPharma, BIAL and Sanofi-Aventis, has received royalties for consulting from UCB Pharma, Alexion Pharmaceuticals, and Sanofi-Aventis, and has received financial research support from Euroimmun, Fresenius Medical Care, Diamed, Alexion Pharmaceuticals, and Novartis Pharma. D.K. received travel grants from GeNeuro and Merck, refund of congress participation fees from GeNeuro, Merck and Servier, consulting fees from Grifols, payment for lectures from Grifols, support for research projects from Teva, and was funded by the Deutsche Forschungsgemeinschaft (DFG). The remaining authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

