Molecular Mechanisms of Reelin in the Enteric Nervous System and the Microbiota-Gut-Brain Axis: Implications for Depression and Antidepressant Therapy
- PMID: 38255890
- PMCID: PMC10815176
- DOI: 10.3390/ijms25020814
Molecular Mechanisms of Reelin in the Enteric Nervous System and the Microbiota-Gut-Brain Axis: Implications for Depression and Antidepressant Therapy
Abstract
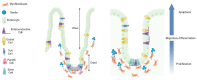
Current pharmacological treatments for depression fail to produce adequate remission in a significant proportion of patients. Increasingly, other systems, such as the microbiome-gut-brain axis, are being looked at as putative novel avenues for depression treatment. Dysbiosis and dysregulation along this axis are highly comorbid with the severity of depression symptoms. The endogenous extracellular matrix protein reelin is present in all intestinal layers as well as in myenteric and submucosal ganglia, and its receptors are also present in the gut. Reelin secretion from subepithelial myofibroblasts regulates cellular migration along the crypt-villus axis in the small intestine and colon. Reelin brain expression is downregulated in mood and psychotic disorders, and reelin injections have fast antidepressant-like effects in animal models of depression. This review seeks to discuss the roles of reelin in the gastrointestinal system and propose a putative role for reelin actions in the microbiota-gut-brain axis in the pathogenesis and treatment of depression, primarily reflecting on alterations in gut epithelial cell renewal and in the clustering of serotonin transporters.
Keywords: antidepressant; crypt–villus axis migration; depression; enteric nervous system; gut–brain axis; microbiota; reelin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Expression and regulation of reelin and its receptors in the enteric nervous system.Mol Cell Neurosci. 2014 Jul;61:23-33. doi: 10.1016/j.mcn.2014.05.001. Epub 2014 May 17. Mol Cell Neurosci. 2014. PMID: 24844606
-
Chinese medicine formula Kai-Xin-San ameliorates depression-like behaviours in chronic unpredictable mild stressed mice by regulating gut microbiota-inflammation-stress system.J Ethnopharmacol. 2020 Oct 28;261:113055. doi: 10.1016/j.jep.2020.113055. Epub 2020 Jun 24. J Ethnopharmacol. 2020. PMID: 32592887
-
Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression.Gastroenterology. 2019 Aug;157(2):507-521.e4. doi: 10.1053/j.gastro.2019.04.022. Epub 2019 May 7. Gastroenterology. 2019. PMID: 31071306 Free PMC article.
-
Enteric nervous system and intestinal epithelial regulation of the gut-brain axis.J Allergy Clin Immunol. 2022 Sep;150(3):513-522. doi: 10.1016/j.jaci.2022.07.015. J Allergy Clin Immunol. 2022. PMID: 36075637 Review.
-
Gut feelings: the microbiota-gut-brain axis on steroids.Am J Physiol Gastrointest Liver Physiol. 2022 Jan 1;322(1):G1-G20. doi: 10.1152/ajpgi.00294.2021. Epub 2021 Nov 3. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34730020 Free PMC article. Review.
Cited by
-
Integrating gut microbiome and neuroplasticity genomics in alcohol use disorder therapy.Hum Genomics. 2025 Jul 11;19(1):78. doi: 10.1186/s40246-025-00793-y. Hum Genomics. 2025. PMID: 40646629 Free PMC article.
-
Trends in research on novel antidepressant treatments.Front Pharmacol. 2025 Jan 27;16:1544795. doi: 10.3389/fphar.2025.1544795. eCollection 2025. Front Pharmacol. 2025. PMID: 39931695 Free PMC article. Review.
-
The efficacy and safety of auricular acupoint therapy on treating functional dyspepsia with insomnia: study protocol for a randomized controlled trial.Front Med (Lausanne). 2025 Mar 12;12:1496502. doi: 10.3389/fmed.2025.1496502. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40144883 Free PMC article.
-
Biomolecular Aspects of Reelin in Neurodegenerative Disorders: An Old Candidate for a New Linkage of the Gut-Brain-Eye Axis.Int J Mol Sci. 2025 Jul 30;26(15):7352. doi: 10.3390/ijms26157352. Int J Mol Sci. 2025. PMID: 40806482 Free PMC article. Review.
-
Unraveling the Role of the Blood-Brain Barrier in the Pathophysiology of Depression: Recent Advances and Future Perspectives.Mol Neurobiol. 2024 Dec;61(12):10398-10447. doi: 10.1007/s12035-024-04205-5. Epub 2024 May 10. Mol Neurobiol. 2024. PMID: 38730081 Review.
References
-
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. - DOI - PubMed
-
- Söderquist F., Syk M., Just D., Kurbalija Novicic Z., Rasmusson A.J., Hellström P.M., Ramklint M., Cunningham J.L. A Cross-Sectional Study of Gastrointestinal Symptoms, Depressive Symptoms and Trait Anxiety in Young Adults. BMC Psychiatry. 2020;20:535. doi: 10.1186/s12888-020-02940-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

