Effect of a New Substance with Pyridoindole Structure on Adult Neurogenesis, Shape of Neurons, and Behavioral Outcomes in a Chronic Mild Stress Model in Rats
- PMID: 38255918
- PMCID: PMC10815319
- DOI: 10.3390/ijms25020845
Effect of a New Substance with Pyridoindole Structure on Adult Neurogenesis, Shape of Neurons, and Behavioral Outcomes in a Chronic Mild Stress Model in Rats
Abstract
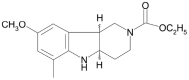
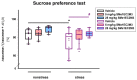
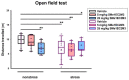
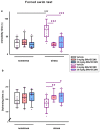
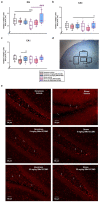
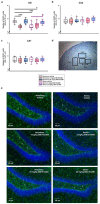
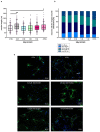
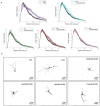
Despite an accumulating number of studies, treatments for depression are currently insufficient. Therefore, the search for new substances with antidepressant potential is very important. In this study, we hypothesized that treatment with a newly synthesized pyridoindole derivative compound SMe1EC2M3 would result in protective and antidepressant-like effects on behavioral outcomes and reverse the impaired adult hippocampal neurogenesis caused by chronic mild stress (CMS). We found that chronic administration of 5 mg/kg and 25 mg/kg SMe1EC2M3 to adult Sprague Dawley rats ameliorated the consequences of CMS on immobility and swimming time in a forced swim test. A slight sedative effect of the highest dose of SMe1EC2M3 in the nonstress group was observed in the open field. SMe1EC2M3 in the highest dose ameliorated CMS-induced decreases in the sucrose preference test. Administration of SMe1EC2M3 significantly increased SOX2-positive cells in the hippocampal dentate gyrus (DG) in CMS compared to control animals. A significant reduction in glial fibrillary acid protein (GFAP)-positive cells in the DG of CMS compared to control animals was observed. Administration of both 5 and 25 mg/kg SMe1EC2M3 significantly increased signal of GFAP-positive cells in the DG of CMS animals. No such effects of SMe1EC2M3 were observed in the cornu ammonis hippocampal area. Additionally, we found that incubation of primary hippocampal neurons in the presence of 1.50 µM SMe1EC2M3 significantly stimulated the length of neurites. Overall, we found that the negative effects of CMS on depression-like behavior are partially reduced by the administration of SMe1EC2M3 and are associated with changes in hippocampal neurogenesis and neuronal differentiation. SMe1EC2M3 represents a potential drug candidate with positive neuroplastic effects and neurogenesis-associated effects in therapeutic approaches to depression.
Keywords: animal model; antidepressants; behavior; immunohistochemistry; major depressive disorder; pyridoindoles; triple reuptake inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Delgado P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry. 2000;61((Suppl. S6)):7–11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

