Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury
- PMID: 38255971
- PMCID: PMC10815150
- DOI: 10.3390/ijms25020897
Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury
Abstract
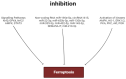
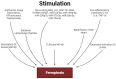
The hospital mortality in patients with ST-segment elevation myocardial infarction (STEMI) is about 6% and has not decreased in recent years. The leading cause of death of these patients is ischemia/reperfusion (I/R) cardiac injury. It is quite obvious that there is an urgent need to create new drugs for the treatment of STEMI based on knowledge about the pathogenesis of I/R cardiac injury, in particular, based on knowledge about the molecular mechanism of ferroptosis. In this study, it was demonstrated that ferroptosis is involved in the development of I/R cardiac injury, antitumor drug-induced cardiomyopathy, diabetic cardiomyopathy, septic cardiomyopathy, and inflammation. There is indirect evidence that ferroptosis participates in stress-induced cardiac injury. The activation of AMPK, PKC, ERK1/2, PI3K, and Akt prevents myocardial ferroptosis. The inhibition of HO-1 alleviates myocardial ferroptosis. The roles of GSK-3β and NOS in the regulation of ferroptosis require further study. The stimulation of Nrf2, STAT3 prevents ferroptosis. The activation of TLR4 and NF-κB promotes ferroptosis of cardiomyocytes. MiR-450b-5p and miR-210-3p can increase the tolerance of cardiomyocytes to hypoxia/reoxygenation through the inhibition of ferroptosis. Circ_0091761 RNA, miR-214-3p, miR-199a-5p, miR-208a/b, miR-375-3p, miR-26b-5p and miR-15a-5p can aggravate myocardial ferroptosis.
Keywords: cardiomyopathy; ferroptosis; heart; ischemia/reperfusion; kinases; microRNAs.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Fabris E., Kilic S., Schellings D.A.A.M., Ten Berg J.M., Kennedy M.W., van Houwelingen K.G., Giannitsis E., Kolkman E., Ottervanger J.P., Hamm C., et al. Long-Term Mortality and Prehospital Tirofiban Treatment in Patients with ST Elevation Myocardial Infarction. Heart. 2017;103:1515–1520. doi: 10.1136/heartjnl-2017-311181. - DOI - PubMed
-
- Olier I., Sirker A., Hildick-Smith D.J.R., Kinnaird T., Ludman P., De Belder M.A., Baumbach A., Byrne J., Rashid M., Curzen N., et al. Association of Different Antiplatelet Therapies with Mortality after Primary Percutaneous Coronary Intervention. Heart. 2018;104:1683–1690. doi: 10.1136/heartjnl-2017-312366. - DOI - PubMed
-
- Megaly M., Pershad A., Glogoza M., Elbadawi A., Omer M., Saad M., Mentias A., Elgendy I., Burke M.N., Capodanno D. Use of Intravascular Imaging in Patients with ST-Segment Elevation Acute Myocardial Infarction. Cardiovasc. Revasc. Med. 2021;30:59–64. doi: 10.1016/j.carrev.2020.09.032. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

