Targeting Innate Immunity in Glioma Therapy
- PMID: 38256021
- PMCID: PMC10815900
- DOI: 10.3390/ijms25020947
Targeting Innate Immunity in Glioma Therapy
Abstract
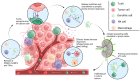
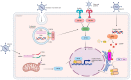
Currently, there is a lack of effective therapies for the majority of glioblastomas (GBMs), the most common and malignant primary brain tumor. While immunotherapies have shown promise in treating various types of cancers, they have had limited success in improving the overall survival of GBM patients. Therefore, advancing GBM treatment requires a deeper understanding of the molecular and cellular mechanisms that cause resistance to immunotherapy. Further insights into the innate immune response are crucial for developing more potent treatments for brain tumors. Our review provides a brief overview of innate immunity. In addition, we provide a discussion of current therapies aimed at boosting the innate immunity in gliomas. These approaches encompass strategies to activate Toll-like receptors, induce stress responses, enhance the innate immune response, leverage interferon type-I therapy, therapeutic antibodies, immune checkpoint antibodies, natural killer (NK) cells, and oncolytic virotherapy, and manipulate the microbiome. Both preclinical and clinical studies indicate that a better understanding of the mechanisms governing the innate immune response in GBM could enhance immunotherapy and reinforce the effects of chemotherapy and radiotherapy. Consequently, a more comprehensive understanding of the innate immune response against cancer should lead to better prognoses and increased overall survival for GBM patients.
Keywords: adaptive therapy; glioma; immunotherapy; innate immunity; virotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Villa S., Manes A., Hostalot C., Capellades J., Costas E., Rosinol O., Sanz C., Rosell R., Balañà C. Short course of radiotherapy and concomitant temozolomide in patients affected with glioblastoma with V–VI prognostic classes: A pilot study. Neuro-Oncology. 2009;11:966–967.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

