Serum-Induced Proliferation of Human Cardiac Stem Cells Is Modulated via TGFβRI/II and SMAD2/3
- PMID: 38256034
- PMCID: PMC10815425
- DOI: 10.3390/ijms25020959
Serum-Induced Proliferation of Human Cardiac Stem Cells Is Modulated via TGFβRI/II and SMAD2/3
Abstract
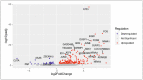
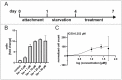
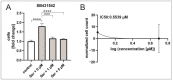
The ageing phenotype is strongly driven by the exhaustion of adult stem cells (ASCs) and the accumulation of senescent cells. Cardiovascular diseases (CVDs) and heart failure (HF) are strongly linked to the ageing phenotype and are the leading cause of death. As the human heart is considered as an organ with low regenerative capacity, treatments targeting the rejuvenation of human cardiac stem cells (hCSCs) are of great interest. In this study, the beneficial effects of human blood serum on proliferation and senescence of hCSCs have been investigated at the molecular level. We show the induction of a proliferation-related gene expression response by human blood serum at the mRNA level. The concurrent differential expression of the TGFβ target and inhibitor genes indicates the participation of TGFβ signalling in this context. Surprisingly, the application of TGFβ1 as well as the inhibition of TGFβ type I and type II receptor (TGFβRI/II) signalling strongly increased the proliferation of hCSCs. Likewise, both human blood serum and TGFβ1 reduced the senescence in hCSCs. The protective effect of serum on senescence in hCSCs was enhanced by simultaneous TGFβRI/II inhibition. These results strongly indicate a dual role of TGFβ signalling in terms of the serum-mediated effects on hCSCs. Further analysis via RNA sequencing (RNA-Seq) revealed the participation of Ras-inactivating genes wherefore a prevention of hyperproliferation upon serum-treatment in hCSCs via TGFβ signalling and Ras-induced senescence is suggested. These insights may improve treatments of heart failure in the future.
Keywords: RNA-Seq; TGFβ1; ageing; human blood serum; human cardiac stem cells; proliferation; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cooper G.M., Cooper G.M. The Cell. 2nd ed. Sinauer Associates; Sunderland, MA, USA: 2000.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

