Impaired Expression of Humanin during Adrenocortical Carcinoma
- PMID: 38256114
- PMCID: PMC10816135
- DOI: 10.3390/ijms25021038
Impaired Expression of Humanin during Adrenocortical Carcinoma
Abstract
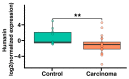
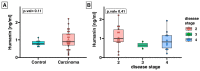
The discovery of mitochondria-derived peptides (MDPs) has provided a new perspective on mitochondrial function. MDPs encoded by mitochondrial DNA (mtDNA) can act as hormone-like peptides, influencing cell survival and proliferation. Among these peptides, humanin has been identified as a crucial factor for maintaining cell survival and preventing cell death under various conditions. Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy that results from adrenal hormone dysfunction. This study aimed to investigate humanin expression in the adrenal tissue and serum of patients with ACC. For the first time, our study revealed significant reduction in the mRNA expression of humanin in patients with ACC compared to healthy controls. However, no significant changes were observed in the serum humanin levels. Interestingly, we identified a positive correlation between patient age and serum humanin levels and a negative correlation between tumor size and LDL levels. While the impaired expression of humanin in patients with ACC may be attributed to mitochondrial dysfunction, an alternative explanation could be related to diminished mitochondrial copy number. Further investigations are warranted to elucidate the intricate relationship among humanin, mitochondrial function, and ACC pathology.
Keywords: ACC; humanin; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Cobb L.J., Lee C., Xiao J., Yen K., Wong R.G., Nakamura H.K., Mehta H.H., Gao Q., Ashur C., Huffman D.M., et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging. 2016;8:796–809. doi: 10.18632/aging.100943. - DOI - PMC - PubMed
-
- Lee C., Zeng J., Drew B.G., Sallam T., Martin-Montalvo A., Wan J., Kim S.J., Mehta H., Hevener A.L., de Cabo R., et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. - DOI - PMC - PubMed
-
- Hashimoto Y., Niikura T., Tajima H., Yasukawa T., Sudo H., Ito Y., Kita Y., Kawasumi M., Kouyama K., Doyu M., et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc. Natl. Acad. Sci. USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. - DOI - PMC - PubMed
-
- Miller B., Kim S.J., Mehta H.H., Cao K., Kumagai H., Thumaty N., Leelaprachakul N., Braniff R.G., Jiao H., Vaughan J., et al. Mitochondrial DNA variation in Alzheimer’s disease reveals a unique microprotein called SHMOOSE. Mol. Psychiatry. 2023;28:1813–1826. doi: 10.1038/s41380-022-01769-3. - DOI - PMC - PubMed
-
- Kienzle L., Bettinazzi S., Choquette T., Brunet M., Khorami H.H., Jacques J.F., Moreau M., Roucou X., Landry C.R., Angers A., et al. A small protein coded within the mitochondrial canonical gene nd4 regulates mitochondrial bioenergetics. BMC Biol. 2023;21:111. doi: 10.1186/s12915-023-01609-y. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

