Invertebrate Immunity, Natural Transplantation Immunity, Somatic and Germ Cell Parasitism, and Transposon Defense
- PMID: 38256145
- PMCID: PMC10815962
- DOI: 10.3390/ijms25021072
Invertebrate Immunity, Natural Transplantation Immunity, Somatic and Germ Cell Parasitism, and Transposon Defense
Abstract
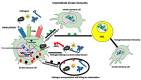
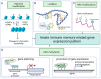
While the vertebrate immune system consists of innate and adaptive branches, invertebrates only have innate immunity. This feature makes them an ideal model system for studying the cellular and molecular mechanisms of innate immunity sensu stricto without reciprocal interferences from adaptive immunity. Although invertebrate immunity is evolutionarily older and a precursor of vertebrate immunity, it is far from simple. Despite lacking lymphocytes and functional immunoglobulin, the invertebrate immune system has many sophisticated mechanisms and features, such as long-term immune memory, which, for decades, have been exclusively attributed to adaptive immunity. In this review, we describe the cellular and molecular aspects of invertebrate immunity, including the epigenetic foundation of innate memory, the transgenerational inheritance of immunity, genetic immunity against invading transposons, the mechanisms of self-recognition, natural transplantation, and germ/somatic cell parasitism.
Keywords: epigenetics; hemocyte; innate immunity; innate memory; invertebrate; transgenerational inheritance; transposons.
Conflict of interest statement
All authors declare that they do not have any conflicts of interest.
Figures

Similar articles
-
Innate Immune Memory in Invertebrate Metazoans: A Critical Appraisal.Front Immunol. 2018 Aug 22;9:1915. doi: 10.3389/fimmu.2018.01915. eCollection 2018. Front Immunol. 2018. PMID: 30186286 Free PMC article. Review.
-
Peculiarities of innate immune memory in crustaceans.Fish Shellfish Immunol. 2020 Sep;104:605-612. doi: 10.1016/j.fsi.2020.06.047. Epub 2020 Jun 30. Fish Shellfish Immunol. 2020. PMID: 32619624 Review.
-
Evolutionary insights into the origin of innate and adaptive immune systems: different shades of grey.Asian Pac J Allergy Immunol. 2014 Mar;32(1):3-15. Asian Pac J Allergy Immunol. 2014. PMID: 24641285 Review.
-
Innate immune memory: towards a better understanding of host defense mechanisms.Curr Opin Immunol. 2014 Aug;29:1-7. doi: 10.1016/j.coi.2014.02.006. Epub 2014 Mar 12. Curr Opin Immunol. 2014. PMID: 24637148 Review.
-
Innate immune memory: An evolutionary perspective.Immunol Rev. 2018 May;283(1):21-40. doi: 10.1111/imr.12647. Immunol Rev. 2018. PMID: 29664574 Review.
Cited by
-
RNA-Seq Analysis Revealed the Virulence Regulatory Network Mediated by the Ferric Uptake Regulator (Fur) in Apostichopus japonicus Pathogenesis Induced by Vibrio splendidus.Microorganisms. 2025 May 22;13(6):1173. doi: 10.3390/microorganisms13061173. Microorganisms. 2025. PMID: 40572061 Free PMC article.
-
Systematic review of innate immune responses against Mycobacterium tuberculosis complex infection in animal models.Front Immunol. 2025 Jan 30;15:1467016. doi: 10.3389/fimmu.2024.1467016. eCollection 2024. Front Immunol. 2025. PMID: 39949719 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

