A Versatile Aldehyde: Ferredoxin Oxidoreductase from the Organic Acid Reducing Thermoanaerobacter sp. Strain X514
- PMID: 38256150
- PMCID: PMC10816221
- DOI: 10.3390/ijms25021077
A Versatile Aldehyde: Ferredoxin Oxidoreductase from the Organic Acid Reducing Thermoanaerobacter sp. Strain X514
Abstract
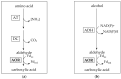
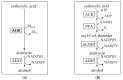
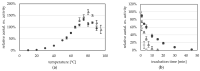
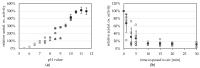
Aldehyde:ferredoxin oxidoreductases (AORs) have been isolated and biochemically-characterized from a handful of anaerobic or facultative aerobic archaea and bacteria. They catalyze the ferredoxin (Fd)-dependent oxidation of aldehydes to acids. Recently, the involvement of AOR in the reduction of organic acids to alcohols with electrons derived from sugar or synthesis gas was demonstrated, with alcohol dehydrogenases (ADHs) carrying out the reduction of the aldehyde to the alcohol (AOR-ADH pathway). Here, we describe the biochemical characterization of an AOR of the thermophilic fermentative bacterium Thermoanaerobacter sp. strain X514 (AORX514). The putative aor gene (Teth514_1380) including a 6x-His-tag was introduced into the genome of the genetically-accessible, related species Thermoanaerobacter kivui. The protein was purified to apparent homogeneity, and indeed revealed AOR activity, as measured by acetaldehyde-dependent ferredoxin reduction. AORX514 was active over a wide temperature (10 to 95 °C) and pH (5.5 to 11.5) range, utilized a wide variety of aldehydes (short and branched-chained, aliphatic, aromatic) and resembles archaeal sensu stricto AORs, as the protein is active in a homodimeric form. The successful, recombinant production of AORX514 in a related, well-characterized and likewise strict anaerobe paves the road towards structure-function analyses of this enzyme and possibly similar oxygen-sensitive or W/Mo-dependent proteins in the future.
Keywords: AOR-ADH pathway; aldehyde:ferredoxin oxidoreductase; ethanol; organic acid reduction; thermoanaerobacter.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Thermoanaerobacter species differ in their potential to reduce organic acids to their corresponding alcohols.Appl Microbiol Biotechnol. 2018 Oct;102(19):8465-8476. doi: 10.1007/s00253-018-9210-3. Epub 2018 Jul 10. Appl Microbiol Biotechnol. 2018. PMID: 29987342
-
Alcohol dehydrogenases AdhE and AdhB with broad substrate ranges are important enzymes for organic acid reduction in Thermoanaerobacter sp. strain X514.Biotechnol Biofuels. 2021 Sep 25;14(1):187. doi: 10.1186/s13068-021-02038-1. Biotechnol Biofuels. 2021. PMID: 34563250 Free PMC article.
-
Purification, characterization, and metabolic function of tungsten-containing aldehyde ferredoxin oxidoreductase from the hyperthermophilic and proteolytic archaeon Thermococcus strain ES-1.J Bacteriol. 1995 Aug;177(16):4757-64. doi: 10.1128/jb.177.16.4757-4764.1995. J Bacteriol. 1995. PMID: 7642503 Free PMC article.
-
The emerging role of aldehyde:ferredoxin oxidoreductases in microbially-catalyzed alcohol production.J Biotechnol. 2019 Dec 20;306:105-117. doi: 10.1016/j.jbiotec.2019.09.005. Epub 2019 Sep 18. J Biotechnol. 2019. PMID: 31541665 Review.
-
Secondary Alcohol Dehydrogenases from Thermoanaerobacter pseudoethanolicus and Thermoanaerobacter brockii as Robust Catalysts.Chembiochem. 2021 Jun 2;22(11):1884-1893. doi: 10.1002/cbic.202100043. Epub 2021 Apr 8. Chembiochem. 2021. PMID: 33594812 Review.
Cited by
-
Obligately Tungsten-Dependent Enzymes─Catalytic Mechanisms, Models and Applications.Biochemistry. 2025 May 20;64(10):2154-2172. doi: 10.1021/acs.biochem.5c00116. Epub 2025 May 5. Biochemistry. 2025. PMID: 40323690 Free PMC article. Review.
References
-
- Mukund S., Adams M.W.W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase: Evidence for its participation in a unique glycolytic pathway. J. Biol. Chem. 1991;266:14208–14216. doi: 10.1016/S0021-9258(18)98669-2. - DOI - PubMed
-
- Huber C., Skopan H., Feicht R., White H., Simon H. Pterin cofactor, substrate specificity, and observations on the kinetics of the reversible tungsten-containing aldehyde oxidoreductase from Clostridium thermoaceticum. Arch. Microbiol. 1995;164:110–118. doi: 10.1007/BF02525316. - DOI
-
- White H., Huber C., Feicht R., Simon H. On a reversible molybdenum-containing aldehyde oxidoreductase from Clostridium formicoaceticum. Arch. Microbiol. 1993;159:244–249. doi: 10.1007/BF00248479. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

