High-Voltage Electrostatic Field Hydrogel Microsphere 3D Culture System Improves Viability and Liver-like Properties of HepG2 Cells
- PMID: 38256154
- PMCID: PMC10816196
- DOI: 10.3390/ijms25021081
High-Voltage Electrostatic Field Hydrogel Microsphere 3D Culture System Improves Viability and Liver-like Properties of HepG2 Cells
Abstract
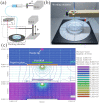
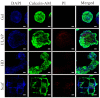
Three-dimensional (3D) hepatocyte models have become a research hotspot for evaluating drug metabolism and hepatotoxicity. Compared to two-dimensional (2D) cultures, 3D cultures are better at mimicking the morphology and microenvironment of hepatocytes in vivo. However, commonly used 3D culture techniques are not suitable for high-throughput drug screening (HTS) due to their high cost, complex handling, and inability to simulate cell-extracellular matrix (ECM) interactions. This article describes a method for rapid and reproducible 3D cell cultures with ECM-cell interactions based on 3D culture instrumentation to provide more efficient HTS. We developed a microsphere preparation based on a high-voltage electrostatic (HVE) field and used sodium alginate- and collagen-based hydrogels as scaffolds for 3D cultures of HepG2 cells. The microsphere-generating device enables the rapid and reproducible preparation of bioactive hydrogel microspheres. This 3D culture system exhibited better cell viability, heterogeneity, and drug-metabolizing activity than 2D and other 3D culture models, and the long-term culture characteristics of this system make it suitable for predicting long-term liver toxicity. This system improves the overall applicability of HepG2 spheroids in safety assessment studies, and this simple and controllable high-throughput-compatible method shows potential for use in drug toxicity screening assays and mechanistic studies.
Keywords: 3D cultures; RNA-seq; hepatocyte; high-voltage electrostatic field; hydrogel; microspheres.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres.Biomaterials. 2017 Jan;115:141-154. doi: 10.1016/j.biomaterials.2016.10.052. Epub 2016 Nov 1. Biomaterials. 2017. PMID: 27889665
-
A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies.Arch Toxicol. 2014 May;88(5):1083-95. doi: 10.1007/s00204-014-1215-9. Epub 2014 Mar 6. Arch Toxicol. 2014. PMID: 24599296
-
Enhanced hepatotoxicity assessment through encapsulated HepG2 spheroids in gelatin hydrogel matrices: Bridging the gap from 2D to 3D culture.Eur J Pharm Biopharm. 2024 Sep;202:114417. doi: 10.1016/j.ejpb.2024.114417. Epub 2024 Jul 14. Eur J Pharm Biopharm. 2024. PMID: 39013493
-
Tissue-Engineered 3D In Vitro Disease Models for High-Throughput Drug Screening.Tissue Eng Regen Med. 2023 Jul;20(4):523-538. doi: 10.1007/s13770-023-00522-3. Epub 2023 Mar 9. Tissue Eng Regen Med. 2023. PMID: 36892736 Free PMC article. Review.
-
3D Cultivation Techniques for Primary Human Hepatocytes.Microarrays (Basel). 2015 Feb 16;4(1):64-83. doi: 10.3390/microarrays4010064. Microarrays (Basel). 2015. PMID: 27600213 Free PMC article. Review.
Cited by
-
Construction of Microsphere Culture System for Human Mesenchymal Stem Cell Aggregates.Int J Mol Sci. 2025 Jul 4;26(13):6435. doi: 10.3390/ijms26136435. Int J Mol Sci. 2025. PMID: 40650211 Free PMC article.
-
Reconstructing the hepatocellular carcinoma microenvironment: the current status and challenges of 3D culture technology.Discov Oncol. 2025 Apr 10;16(1):506. doi: 10.1007/s12672-025-02290-z. Discov Oncol. 2025. PMID: 40208520 Free PMC article. Review.
References
-
- Blackford S.J.I., Yu T.T.L., Norman M.D.A., Syanda A.M., Manolakakis M., Lachowski D., Yan Z., Guo Y., Garitta E., Riccio F., et al. RGD density along with substrate stiffness regulate hPSC hepatocyte functionality through YAP signalling. Biomaterials. 2023;293:121982. doi: 10.1016/j.biomaterials.2022.121982. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

