NAD+ Precursors Reverse Experimental Diabetic Neuropathy in Mice
- PMID: 38256175
- PMCID: PMC10816262
- DOI: 10.3390/ijms25021102
NAD+ Precursors Reverse Experimental Diabetic Neuropathy in Mice
Abstract
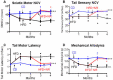
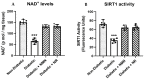
Abnormal NAD+ signaling has been implicated in axonal degeneration in diabetic peripheral neuropathy (DPN). We hypothesized that supplementing NAD+ precursors could alleviate DPN symptoms through increasing the NAD+ levels and activating the sirtuin-1 (SIRT1) protein. To test this, we exposed cultured Dorsal Root Ganglion neurons (DRGs) to Nicotinamide Riboside (NR) or Nicotinamide Mononucleotide (NMN), which increased the levels of NAD+, the SIRT1 protein, and the deacetylation activity that is associated with increased neurite growth. A SIRT1 inhibitor blocked the neurite growth induced via NR or NMN. We then induced neuropathy in C57BL6 mice with streptozotocin (STZ) or a high fat diet (HFD) and administered NR or NMN for two months. Both the STZ and HFD mice developed neuropathy, which was reversed through the NR or NMN administration: sensory function improved, nerve conduction velocities normalized, and intraepidermal nerve fibers were restored. The NAD+ levels and SIRT1 activity were reduced in the DRGs from diabetic mice but were preserved with the NR or NMN treatment. We also tested the effect of NR or NMN administration in mice that overexpress the SIRT1 protein in neurons (nSIRT1 OE) and found no additional benefit from the addition of the drug. These findings suggest that supplementing with NAD+ precursors or activating SIRT1 may be a promising treatment for DPN.
Keywords: NAD+; NEDD4-1; SIRT1; diabetic neuropathy; high fat diet; mitochondria; streptozotocin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Chandrasekaran K., Najimi N., Sagi A.R., Yarlagadda S., Salimian M., Arvas M.I., Hedayat A.F., Kevas Y., Kadakia A., Russell J.W. NAD(+) Precursors Repair Mitochondrial Function in Diabetes and Prevent Experimental Diabetic Neuropathy. Int. J. Mol. Sci. 2022;23:4887. doi: 10.3390/ijms23094887. - DOI - PMC - PubMed
-
- Chandrasekaran K., Choi J., Arvas M.I., Salimian M., Singh S., Xu S., Gullapalli R.P., Kristian T., Russell J.W. Nicotinamide Mononucleotide Administration Prevents Experimental Diabetes-Induced Cognitive Impairment and Loss of Hippocampal Neurons. Int. J. Mol. Sci. 2020;21:3756. doi: 10.3390/ijms21113756. - DOI - PMC - PubMed
-
- Chandrasekaran K., Anjaneyulu M., Choi J., Kumar P., Salimian M., Ho C.Y., Russell J.W. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD(+)-dependent SIRT1-PGC-1alpha-TFAM pathway. Int. Rev. Neurobiol. 2016;145:177–209. doi: 10.1016/bs.irn.2019.04.002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

