Transgenerational Transmission of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Effects in Human Granulosa Cells: The Role of MicroRNAs
- PMID: 38256218
- PMCID: PMC10816780
- DOI: 10.3390/ijms25021144
Transgenerational Transmission of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Effects in Human Granulosa Cells: The Role of MicroRNAs
Abstract
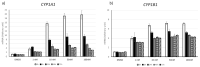
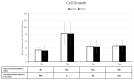
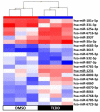
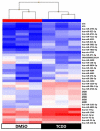
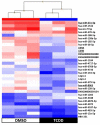
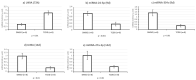
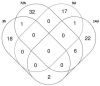
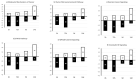
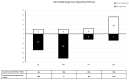
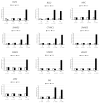
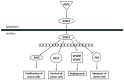
Endocrine-disrupting chemicals (EDCs) might contribute to the increase in female-specific cancers in Western countries. 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) is considered the "prototypical toxicant" to study EDCs' effects on reproductive health. Epigenetic regulation by small noncoding RNAs (sncRNAs), such as microRNAs (miRNA), is crucial for controlling cancer development. The aim of this study was to analyze transcriptional activity and sncRNA expression changes in the KGN cell line after acute (3 h) and chronic (72 h) exposure to 10 nM TCDD in order to determine whether sncRNAs' deregulation may contribute to transmitting TCDD effects to the subsequent cell generations (day 9 and day 14 after chronic exposure). Using Affymetrix GeneChip miRNA 4.0 arrays, 109 sncRNAs were found to be differentially expressed (fold change < -2 or >2; p-value < 0.05) between cells exposed or not (control) to TCDD for 3 h and 72 h and on day 9 and day 14 after chronic exposure. Ingenuity Pathway Analysis predicted that following the acute and chronic exposure of KGN cells, sncRNAs linked to cellular development, growth and proliferation were downregulated, and those linked to cancer promotion were upregulated on day 9 and day 14. These results indicated that TCDD-induced sncRNA dysregulation may have transgenerational cancer-promoting effects.
Keywords: TCDD; cancers; dioxin; endocrine-disrupting chemicals (EDCs); epigenetics; granulosa cells; miRNA; transgenerational transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Del Pup L., Mantovani A., Luce A., Cavaliere C., Facchini G., Di Francia R., Caraglia M., Berretta M. Endocrine disruptors and female cancer: Informing the patients (Review) Oncol. Rep. 2015;34:3–11. - PubMed
-
- Moran F.M., Conley A.J., Corbin C.J., Enan E., VandeVoort C., Overstreet J.W., Lasley B.L. 2,3,7,8-tetrachlorodibenzo-p-dioxin decreases estradiol production without altering the enzyme activity of cytochrome P450 aromatase of human luteinized granulosa cells in vitro. Biol. Reprod. 2000;62:1102–1108. doi: 10.1095/biolreprod62.4.1102. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

