The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma
- PMID: 38256223
- PMCID: PMC10816929
- DOI: 10.3390/ijms25021150
The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma
Abstract
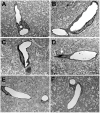
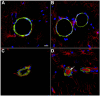
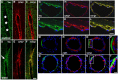
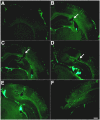
Blast-induced neurotrauma has received much attention over the past decade. Vascular injury occurs early following blast exposure. Indeed, in animal models that approximate human mild traumatic brain injury or subclinical blast exposure, vascular pathology can occur in the presence of a normal neuropil, suggesting that the vasculature is particularly vulnerable. Brain endothelial cells and their supporting glial and neuronal elements constitute a neurovascular unit (NVU). Blast injury disrupts gliovascular and neurovascular connections in addition to damaging endothelial cells, basal laminae, smooth muscle cells, and pericytes as well as causing extracellular matrix reorganization. Perivascular pathology becomes associated with phospho-tau accumulation and chronic perivascular inflammation. Disruption of the NVU should impact activity-dependent regulation of cerebral blood flow, blood-brain barrier permeability, and glymphatic flow. Here, we review work in an animal model of low-level blast injury that we have been studying for over a decade. We review work supporting the NVU as a locus of low-level blast injury. We integrate our findings with those from other laboratories studying similar models that collectively suggest that damage to astrocytes and other perivascular cells as well as chronic immune activation play a role in the persistent neurobehavioral changes that follow blast injury.
Keywords: animal models; astrocytes; blast; inflammation; traumatic brain injury; vascular pathology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Tanielian T., Jaycox L.H. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Rand Corporation; Santa Monica, CA, USA: 2008.
-
- DePalma R.G. Combat TBI: History, Epidemiology and Injury Modes. In: Kobeissy F., editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press; Boca Raton, FL, USA: 2015. pp. 5–14.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

