Gas Phase Transformations in Carbon-11 Chemistry
- PMID: 38256240
- PMCID: PMC10816134
- DOI: 10.3390/ijms25021167
Gas Phase Transformations in Carbon-11 Chemistry
Abstract
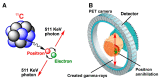
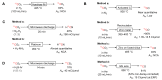
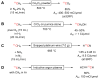
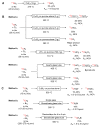
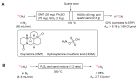
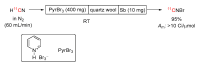
The short-lived positron-emitter carbon-11 (t1/2 = 20.4 min; β+, 99.8%) is prominent for labeling tracers for use in biomedical research with positron emission tomography (PET). Carbon-11 is produced for this purpose with a cyclotron, nowadays almost exclusively by the 14N(p,α)11C nuclear reaction, either on nitrogen containing a low concentration of oxygen (0.1-0.5%) or hydrogen (~5%) to produce [11C]carbon dioxide or [11C]methane, respectively. These primary radioactive products can be produced in high yields and with high molar activities. However, only [11C]carbon dioxide has some utility for directly labeling PET tracers. Primary products are required to be converted rapidly and efficiently into secondary labeling synthons to provide versatile radiochemistry for labeling diverse tracer chemotypes at molecular positions of choice. This review surveys known gas phase transformations of carbon-11 and summarizes the important roles that many of these transformations now play for producing a broad range of labeling synthons in carbon-11 chemistry.
Keywords: PET; carbon-11; catalysts; gas phase; on-line processes; radiochemistry; radiotracer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



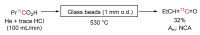
References
-
- Ferrieri R.A., Wolf A.P. The chemistry of positron emitting nucleogenic (hot) atoms with regard to preparation of labelled compounds of practical utility. Radiochim. Acta. 1983;34:69–84. doi: 10.1524/ract.1983.34.12.69. - DOI
-
- Ferrieri R.A. Handbook of Radiopharmaceuticals—Radiochemistry and Applications. John Wiley & Sons; Hoboken, NJ, USA: 2002. Production and Application of Synthetic Precursors Labeled with Carbon-11 and Fluorine-18; pp. 229–282. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

