Effectiveness, Tolerability, and Drug Survival of Risankizumab in a Real-World Setting: A Three-Year Retrospective Multicenter Study-IL PSO (ITALIAN LANDSCAPE PSORIASIS)
- PMID: 38256629
- PMCID: PMC10816779
- DOI: 10.3390/jcm13020495
Effectiveness, Tolerability, and Drug Survival of Risankizumab in a Real-World Setting: A Three-Year Retrospective Multicenter Study-IL PSO (ITALIAN LANDSCAPE PSORIASIS)
Abstract
Background: Risankizumab is a humanized monoclonal antibody that selectively inhibits interleukin-23. It has been approved for moderate-to-severe plaque psoriasis and has shown efficacy and safety in clinical trials and real-world experiences. This study aimed to evaluate the long-term effectiveness, safety, and drug survival of risankizumab in a real-life setting.
Materials and methods: We included patients treated with risankizumab from January 2019 to February 2023. A Psoriasis Area and Severity Index score (PASI) was collected at weeks 0, 16, 28, 52, 104, and 156, when available. The occurrence of any adverse events was recorded at each visit.
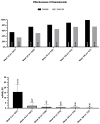
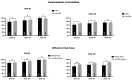
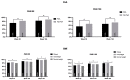
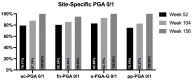
Results: We enrolled 1047 patients. At week 52, a ≥90% improvement in PASI was observed in 81.44% of patients, with a continuous improvement throughout the study (88.99% and 99.07% at weeks 104 and 156, respectively). After three years of treatment, all patients involving the scalp, palms/soles, and genitalia and 95% of patients with nail psoriasis achieved a complete or almost complete skin clearance. No significant safety findings were observed, and 90.73% of the patients were still on treatment after 36 months.
Conclusions: This study supports the long-term effectiveness and safety of risankizumab in a real-world setting, even in patients involving difficult-to-treat areas.
Keywords: biological therapy; psoriasis; real-world evidence; risankizumab.
Conflict of interest statement
L. Gargiulo was a consultant for Almirall. L. Ibba was a consultant for Almirall. P. Malagoli was a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. A. Balato received honoraria for participation in advisory boards, meetings, or as a speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. F. Bardazzi was a consultant advisor and clinical study investigator for Eli Lilly, Abbvie, Novartis, Leo Pharma, Sandoz, Bristol Myers, Abiogen-Pharma, Celgene, and Janssen. M. Burlando acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, and UCB Pharma. C. G. Carrera served as a board participant or speaker for Abbvie, Lilly, Janssen, Novartis, Celgene, Almirall, and Leopharma. G. Damiani served as consultant and/or speaker for AbbVie, Almirall, Bristol-Meyers Squibb, LeoPharma, Novartis, Pfizer, Sanofi, and UCB and received unrestricted grants from Pfizer and Almirall. P. Dapavo was a speaker for Novartis, Abbvie, Sanofi, UCB, Janssen, Lilly, and LeoPharma. G. Fabbrocini acted as a speaker or consultant for Abbvie, Amgen, Eli Lilly, Janssen, Leo-Pharma, Almirall, Novartis, Sanofi, and UCB. F. M. Gaiani acted as a speaker or consultant for Novartis, Abbvie, Eli Lilly, Celgene, LeoPharma, and Almirall. G. Girolomoni served as consultant and/or speaker for AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Eli-Lilly, LeoPharma, Novartis, Pfizer, Samsung, Sanofi, and UCB. C. Guarneri was a scientific consultant/speaker/clinical study investigator for Abbvie, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Almirall, and LEO Pharma. C. Lasagni declares a conflict of interest with Abbvie, Novartis, Lilly, and Almirall. F. Loconsole served on advisory boards and/or received honoraria for lectures from Abbvie, Janssen-Cilag, Novartis, Lilly, and Sanofi. A. V. Marzano reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer Ingelheim, Novartis, Pfizer, Sanofi, and UCB. M. Megna acted as a speaker or consultant for Abbvie, Eli Lilly, Janssen, Leo-Pharma, UCB, and Novartis. F. Sampogna served as a consultant for AbbVie. M. Valenti was a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie, and Boehringer Ingelheim. A. Costanzo served as an advisory board member, and consultant and received fees and speaker honoraria or participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi served on advisory boards and received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen, and Boehringer Ingelheim. F. Amoruso, G. Argenziano, V. Dini, C. Franchi, M. Maurelli, D. Orsini, and M. Travaglini have nothing to declare.
Figures

References
-
- Sbidian E., Chaimani A., Garcia-Doval I., Do G., Hua C., Mazaud C., Droitcourt C., Hughes C., Ingram J.R., Naldi L., et al. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2017;12:CD011535. doi: 10.1002/14651858.CD011535.pub2. - DOI - PMC - PubMed
-
- Gisondi P., Geat D., Conti A., Dapavo P., Piaserico S., De Simone C., Bianchi L., Costanzo A., Malagoli P., Malara G., et al. TNF-α inhibitors biosimilars as first line systemic treatment for moderate-to-severe chronic plaque psoriasis. Expert. Rev. Clin. Immunol. 2020;16:591–598. doi: 10.1080/1744666X.2020.1771182. - DOI - PubMed

