Controversial Past, Splendid Present, Unpredictable Future: A Brief Review of Alzheimer Disease History
- PMID: 38256670
- PMCID: PMC10816332
- DOI: 10.3390/jcm13020536
Controversial Past, Splendid Present, Unpredictable Future: A Brief Review of Alzheimer Disease History
Abstract
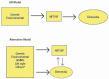
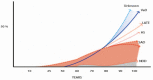
Background: The concept of Alzheimer disease (AD)-since its histological discovery by Alzheimer to the present day-has undergone substantial modifications. Methods: We conducted a classical narrative review of this field with a bibliography selection (giving preference to Medline best match). Results: The following subjects are reviewed and discussed: Alzheimer's discovery, Kraepelin's creation of a new disease that was a rare condition until the 1970's, the growing interest and investment in AD as a major killer in a society with a large elderly population in the second half of the 20th century, the consolidation of the AD clinicopathological model, and the modern AD nosology based on the dominant amyloid hypothesis among many others. In the 21st century, the development of AD biomarkers has supported a novel biological definition of AD, although the proposed therapies have failed to cure this disease. The incidence of dementia/AD has shown a decrease in affluent countries (possibly due to control of risk factors), and mixed dementia has been established as the most frequent etiology in the oldest old. Conclusions: The current concept of AD lacks unanimity. Many hypotheses attempt to explain its complex physiopathology entwined with aging, and the dominant amyloid cascade has yielded poor therapeutic results. The reduction in the incidence of dementia/AD appears promising but it should be confirmed in the future. A reevaluation of the AD concept is also necessary.
Keywords: Alzheimer disease; elderly dementia; future; hypothesis; nosological concept; past history; pathology; physiopathology; prevention; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
What is Alzheimer's disease? An analysis of nosological perspectives from the 20th and 21st centuries.Eur J Neurol. 2024 Nov;31(11):e16302. doi: 10.1111/ene.16302. Epub 2024 Apr 15. Eur J Neurol. 2024. PMID: 38618742 Free PMC article. Review.
-
Alzheimer's and dementia in the oldest-old: a century of challenges.Curr Alzheimer Res. 2006 Dec;3(5):411-9. doi: 10.2174/156720506779025233. Curr Alzheimer Res. 2006. PMID: 17168640 Free PMC article. Review.
-
[Alzheimer's disease from Auguste Deter to the present : Progress, disappointments and open questions].Z Gerontol Geriatr. 2017 Oct;50(7):576-587. doi: 10.1007/s00391-017-1307-2. Epub 2017 Sep 18. Z Gerontol Geriatr. 2017. PMID: 28924872 Review. German.
-
Application of the NIA-AA Research Framework: Towards a Biological Definition of Alzheimer's Disease Using Cerebrospinal Fluid Biomarkers in the AIBL Study.J Prev Alzheimers Dis. 2019;6(4):248-255. doi: 10.14283/jpad.2019.25. J Prev Alzheimers Dis. 2019. PMID: 31686097
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Cognitive synaptopathy: synaptic and dendritic spine dysfunction in age-related cognitive disorders.Front Aging Neurosci. 2024 Oct 3;16:1476909. doi: 10.3389/fnagi.2024.1476909. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39420927 Free PMC article. Review.
-
CNEURO-201, an Anti-amyloidogenic Agent and σ1-Receptor Agonist, Improves Cognition in the 3xTg Mouse Model of Alzheimer's Disease by Multiple Actions in the Pathology.Int J Mol Sci. 2025 Feb 3;26(3):1301. doi: 10.3390/ijms26031301. Int J Mol Sci. 2025. PMID: 39941068 Free PMC article.
-
Dysregulation of cerebral perfusion dynamics is associated with Alzheimer's disease.Alzheimers Dement (Amst). 2025 Jul 18;17(3):e70134. doi: 10.1002/dad2.70134. eCollection 2025 Jul-Sep. Alzheimers Dement (Amst). 2025. PMID: 40689334 Free PMC article.
-
Neuropsychological Assessment for Early Detection and Diagnosis of Dementia: Current Knowledge and New Insights.J Clin Med. 2024 Jun 12;13(12):3442. doi: 10.3390/jcm13123442. J Clin Med. 2024. PMID: 38929971 Free PMC article. Review.
-
Deferasirox Derivatives as Inhibitors of Kallikrein-Related Peptidases Associated to Neurodegenerative Diseases.ChemMedChem. 2025 Jul 1;20(13):e202500187. doi: 10.1002/cmdc.202500187. Epub 2025 Apr 24. ChemMedChem. 2025. PMID: 40192482 Free PMC article.
References
-
- Signoret J.L., Hauw J. Maladie d’Alzheimer et Autres Démences. Flammarion; Paris, France: 1991.
-
- Halpert B.P. Development of the term “senility” as a medical diagnosis. Minn. Med. 1983;66:421–424. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

