Investigation of Epilobium hirsutum L. Optimized Extract's Anti-Inflammatory and Antitumor Potential
- PMID: 38256751
- PMCID: PMC10819739
- DOI: 10.3390/plants13020198
Investigation of Epilobium hirsutum L. Optimized Extract's Anti-Inflammatory and Antitumor Potential
Abstract
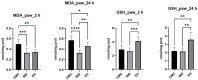
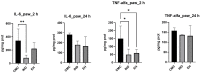
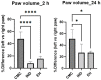
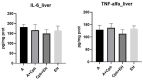
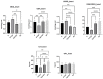
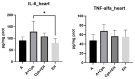
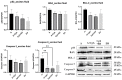
Epilobium hirsutum L., commonly known as hairy willowherb, is a perennial herbaceous plant native to Europe and Asia. In Romania, the Epilobium genus includes 17 species that are used in folk medicine for various purposes. This study aimed to investigate the anti-inflammatory and antitumor potential of the optimized extract of Epilobium hirsutum (EH) in animal models. The first study investigated the anti-inflammatory properties of EH optimized extract and the model used was carrageenan-induced paw inflammation. Wistar rats were divided into three groups: negative control, positive control treated with indomethacin, and a group treated with the extract. Oxidative stress markers, cytokine levels, and protein expressions were assessed. The extract demonstrated anti-inflammatory properties comparable to those of the control group. In the second study, the antitumor effects of the extract were assessed using the tumor model of Ehrlich ascites carcinoma. Swiss albino mice with Ehrlich ascites were divided into four groups: negative, positive treated with cyclophosphamide (Cph), Group 3 treated with Cph and EH optimized extract, and Group 4 treated with extract alone. Samples from the ascites fluid, liver, and heart were analyzed to evaluate oxidative stress, inflammation, and cancer markers. The extract showed a reduction in tumor-associated inflammation and oxidative stress. Overall, the EH optimized extract exhibited promising anti-inflammatory and antitumor effects in the animal models studied. These findings suggest its potential as a natural adjuvant therapeutic agent for addressing inflammation and oxidative stress induced by different pathologies.
Keywords: Epilobium hirsutum; Western Blot analysis; acute rat paw inflammation; anti-inflammatory activity; antioxidant potential; optimized extract.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
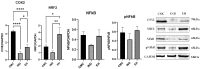



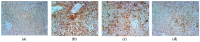


References
-
- Bernstein N., Akram M., Daniyal M., Koltai H., Fridlender M., Gorelick J. Advances in Agronomy. Volume 150. Elsevier; Amsterdam, The Netherlands: 2018. Antiinflammatory Potential of Medicinal Plants: A Source for Therapeutic Secondary Metabolites; pp. 131–183.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

