Affinity-Based Magnetic Nanoparticle Development for Cancer Stem Cell Isolation
- PMID: 38256995
- PMCID: PMC10818538
- DOI: 10.3390/polym16020196
Affinity-Based Magnetic Nanoparticle Development for Cancer Stem Cell Isolation
Abstract
Cancer is still the leading cause of death in the world despite the developing research and treatment opportunities. Failure of these treatments is generally associated with cancer stem cells (CSCs), which cause metastasis and are defined by their resistance to radio- and chemotherapy. Although known stem cell isolation methods are not sufficient for CSC isolation, they also bring a burden in terms of cost. The aim of this study is to develop a high-efficiency, low-cost, specific method for cancer stem cell isolation with magnetic functional nanoparticles. This study, unlike the stem cell isolation techniques (MACS, FACS) used today, was aimed to isolate cancer stem cells (separation of CD133+ cells) with nanoparticles with specific affinity and modification properties. For this purpose, affinity-based magnetic nanoparticles were synthesized and characterized by providing surface activity and chemical reactivity, as well as making surface modifications necessary for both lectin affinity and metal affinity interactions. In the other part of the study, synthesized and characterized functional polymeric magnetic nanoparticles were used for the isolation of CSC from the human osteosarcoma cancer cell line (SAOS-2) with a cancer stem cell subpopulation bearing the CD133 surface marker. The success and efficiency of separation after stem cell isolation were evaluated via the MACS and FACS methods. As a result, when the His-graft-mg-p(HEMA) nanoparticle was used at a concentration of 0.1 µg/mL for 106 and 108 cells, superior separation efficiency to commercial microbeads was obtained.
Keywords: affinity interactions; cancer stem cell; magnetic nanoparticle; stem cell isolation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
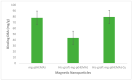
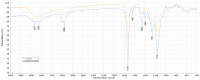

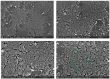
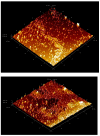
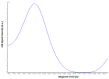
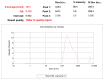

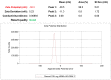
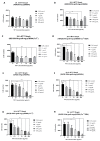


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

