Grafting Starch with Acrylic Acid and Fenton's Initiator: The Selectivity Challenge
- PMID: 38257054
- PMCID: PMC10818371
- DOI: 10.3390/polym16020255
Grafting Starch with Acrylic Acid and Fenton's Initiator: The Selectivity Challenge
Abstract
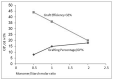
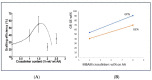
Through the graft polymerization of acrylic monomers onto starch, materials with interesting new properties can be synthesized. Fenton's chemistry, Fe2+/H2O2, is considered to be attractive for the initiation of graft polymerization with the monomer acrylic acid since it is cheap and reacts quickly at ambient conditions and should therefore be easy to scale up. However, the selectivity of the grafting versus the homopolymerization reaction poses a challenge with this monomer and this type of initiator. In the present review paper, we investigate why data from the literature on grafting systems with other monomers and initiation systems tend to show higher graft selectivity. A scheme is presented, based on reaction engineering principles, that supports an explanation for these observed differences. It is found that more selective activation of starch is a factor, but perhaps even more important is a low monomer-to-starch ratio at the starting sites of graft reactions. Since water is the most common solvent, monomers that are less water-soluble have an advantage in this respect. Based on the proposed scheme, methods to improve the graft selectivity with Fenton's initiator and acrylic acid are evaluated. Most promising appears to be a method of gradual monomer dosage. With gelatinized cassava starch in a batch reactor, both the grafting percentage (17 => 29%) and graft selectivity (18 => 31%) could be improved. This can be considered a principal breakthrough. Still, more research and development would be needed to refine the method and to implement the idea in a continuous reactor at a larger scale.
Keywords: Fenton’s initiator; acrylic acid monomer; dedicated monomer dosage; graft selectivity; graphical representation of different grafting systems; methods to improve selectivity; starch graft copolymerization; water solubility of the monomer as a key factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
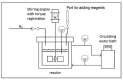
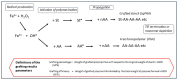


 represents high energy radiation, ● = radical state.
represents high energy radiation, ● = radical state.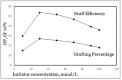



Similar articles
-
Improved homopolymer separation to enable the application of 1H NMR and HPLC for the determination of the reaction parameters of the graft copolymerization of acrylic acid onto starch.Carbohydr Res. 2013 Apr 5;370:38-45. doi: 10.1016/j.carres.2013.01.017. Epub 2013 Jan 31. Carbohydr Res. 2013. PMID: 23435285
-
Graft copolymerization of acrylic acid to cassava starch--evaluation of the influences of process parameters by an experimental design method.Carbohydr Polym. 2012 Nov 6;90(4):1522-9. doi: 10.1016/j.carbpol.2012.07.024. Epub 2012 Jul 20. Carbohydr Polym. 2012. PMID: 22944411
-
The grafting of acrylic acid onto biosorbents: effect of plant components and initiator concentration.Carbohydr Polym. 2012 Sep 1;90(1):201-9. doi: 10.1016/j.carbpol.2012.05.024. Epub 2012 May 11. Carbohydr Polym. 2012. PMID: 24751031
-
Water absorption, retention and the swelling characteristics of cassava starch grafted with polyacrylic acid.Carbohydr Polym. 2014 Mar 15;103:325-32. doi: 10.1016/j.carbpol.2013.12.056. Epub 2013 Dec 22. Carbohydr Polym. 2014. PMID: 24528736
-
On the role of oil-soluble initiators in the radical polymerization of micellar systems.Adv Colloid Interface Sci. 2001 May 25;91(2):295-334. doi: 10.1016/s0001-8686(99)00036-6. Adv Colloid Interface Sci. 2001. PMID: 11392358 Review.
Cited by
-
Ferroptosis and epilepsy: bidirectional pathogenic links and therapeutic implications.Front Neurol. 2025 Jul 30;16:1635441. doi: 10.3389/fneur.2025.1635441. eCollection 2025. Front Neurol. 2025. PMID: 40808923 Free PMC article. Review.
-
Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels.Molecules. 2024 Oct 12;29(20):4835. doi: 10.3390/molecules29204835. Molecules. 2024. PMID: 39459203 Free PMC article. Review.
-
Strategies and Methodologies for Improving Toughness of Starch Films.Foods. 2024 Dec 13;13(24):4036. doi: 10.3390/foods13244036. Foods. 2024. PMID: 39766978 Free PMC article. Review.
References
-
- Meimoun J., Wiatz V., Saint-Loup R., Julien Parcq J., Favrelle A., Bonnet F., Zinck P. Modification of starch by graft copolymerization. Starch/Stärke. 2018;70:1600351. doi: 10.1002/star.201600351. - DOI
-
- Lele V.V., Kumari S., Niju H. Syntheses, Characterization and Applications of Graft Copolymers of Sago Starch—A Review. Starch/Stärke. 2018;70:1700133. doi: 10.1002/star.201700133. - DOI
-
- Athawale V.D., Rathi S.C. Graft Polymerization: Starch as a Model Substrate. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1999;39:445–480. doi: 10.1081/MC-100101424. - DOI
-
- Fanta G.F., Doane W.M. Grafted Starches. In: Wurzburg O.B., editor. Modified Starches: Properties and Uses. CRC Press; Boca Raton, FL, USA: 1986.
-
- Witono J.R. Ph.D. Thesis. Chemical Engineering Dept., University of Groningen; Groningen, The Netherlands: 2012. New Materials by Grafting of Acrylic Acid onto Cassava Starch.
Publication types
LinkOut - more resources
Full Text Sources

