Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice
- PMID: 38257140
- PMCID: PMC10818793
- DOI: 10.3390/nu16020248
Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice
Abstract
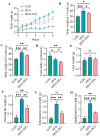
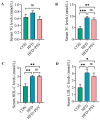
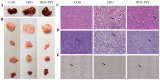
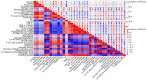
p-Synephrine is a common alkaloid widely distributed in citrus fruits. However, the effects of p-synephrine on the metabolic profiles of individuals with energy abnormalities are still unclear. In the study, we investigated the effect of p-synephrine on energy homeostasis and metabolic profiles using a high fat diet (HFD)-induced mouse model. We found that p-synephrine inhibited the gain in body weight, liver weight and white adipose tissues weight induced by HFD. p-Synephrine supplementation also reduced levels of serum total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) but not to a statistically significant degree. Histological analysis showed that HFD induced excessive lipid accumulation and glycogen loss in the liver and adipocyte enlargement in perirenal fat tissue, while p-synephrine supplementation reversed the changes induced by HFD. Moreover, HFD feeding significantly increased mRNA expression levels of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) and reduced the mRNA expression level of interleukin-10 (IL-10) compared to the control group, while p-synephrine supplementation significantly reversed these HFD-induced changes. Liver and serum metabolomic analysis showed that p-synephrine supplementation significantly altered small molecule metabolites in liver and serum in HFD mice and that the changes were closely associated with improvement of energy homeostasis. Notably, amino acid metabolism pathways, both in liver and serum samples, were significantly enriched. Our study suggests that p-synephrine improves energy homeostasis probably by regulating amino acid metabolism in HFD mice, which provides a novel insight into the action mechanism of p-synephrine modulating energy homeostasis.
Keywords: HFD; amino acid; citrus; energy homeostasis; metabolome; p-synephrine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
p-Synephrine: an overview of physicochemical properties, toxicity, biological and pharmacological activity.EXCLI J. 2025 Mar 5;24:381-400. doi: 10.17179/excli2024-8088. eCollection 2025. EXCLI J. 2025. PMID: 40376440 Free PMC article. Review.
-
Fish Oil Supplementation Alleviates the Altered Lipid Homeostasis in Blood, Liver, and Adipose Tissues in High-Fat Diet-Fed Rats.J Agric Food Chem. 2018 Apr 25;66(16):4118-4128. doi: 10.1021/acs.jafc.8b00529. Epub 2018 Apr 16. J Agric Food Chem. 2018. PMID: 29627983
-
Immature Citrus reticulata Extract Promotes Browning of Beige Adipocytes in High-Fat Diet-Induced C57BL/6 Mice.J Agric Food Chem. 2018 Sep 19;66(37):9697-9703. doi: 10.1021/acs.jafc.8b02719. Epub 2018 Sep 10. J Agric Food Chem. 2018. PMID: 30146891
-
[Effect of diosgenin on mTOR/FASN/HIF-1α/VEGFA expression in rats with non-alcoholic fatty liver disease].Zhongguo Zhong Yao Za Zhi. 2023 Apr;48(7):1760-1769. doi: 10.19540/j.cnki.cjcmm.20221123.401. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37282950 Chinese.
-
[Intervention of Catalpol on High-fat Diet Induced Nonalcoholic Fatty Liver Disease in Mice].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019 Dec 30;41(6):746-755. doi: 10.3881/j.issn.1000-503X.11110. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019. PMID: 31907123 Chinese.
Cited by
-
p-Synephrine: an overview of physicochemical properties, toxicity, biological and pharmacological activity.EXCLI J. 2025 Mar 5;24:381-400. doi: 10.17179/excli2024-8088. eCollection 2025. EXCLI J. 2025. PMID: 40376440 Free PMC article. Review.
-
Untargeted Metabolomics Reveals Key Differences Between Yak, Buffalo, and Cow Colostrum Based on UHPLC-ESI-MS/MS.Foods. 2025 Jan 13;14(2):232. doi: 10.3390/foods14020232. Foods. 2025. PMID: 39856898 Free PMC article.
References
-
- Jauregibeitia I., Portune K., Rica I., Tueros I., Velasco O., Grau G., Trebolazabala N., Castaño L., Larocca A.V., Ferreri C., et al. Fatty Acid Profile of Mature Red Blood Cell Membranes and Dietary Intake as a New Approach to Characterize Children with Overweight and Obesity. Nutrients. 2020;12:3446. doi: 10.3390/nu12113446. - DOI - PMC - PubMed
-
- Kumar V., Kaur R., Aggarwal P., Singh G. Underutilized Citrus Species: An Insight of Their Nutraceutical Potential and Importance for the Development of Functional Food. Sci. Hortic. 2022;296:110909. doi: 10.1016/j.scienta.2022.110909. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

