Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds
- PMID: 38257209
- PMCID: PMC10819569
- DOI: 10.3390/molecules29020296
Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds
Abstract
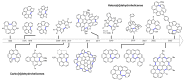
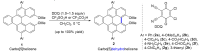
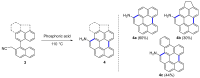
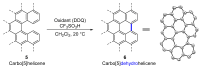
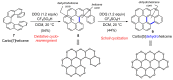
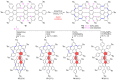
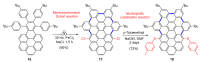
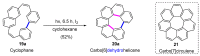
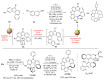
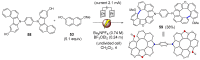
Dehydrohelicene-based molecules stand out as highly promising scaffolds and captivating chiroptical materials, characterized by their unique chirality. Their quasi-helical π-conjugated molecular architecture, featuring successively ortho-annulated aromatic rings, endows them with remarkable thermal stability and optical properties. Over the past decade, diverse approaches have emerged for synthesizing these scaffolds, reinvigorating this field, with anticipated increased attention in the coming years. This review provides a comprehensive overview of the historical evolution of dehydrohelicene chemistry since the pioneering work of Zander and Franke in 1969 and highlights recent advancements in the synthesis of various molecules incorporating dehydrohelicene motifs. We elucidate the intriguing structural features and optical merits of these molecules, occasionally drawing comparisons with their helicene or circulene analogs to underscore the significance of the bond between the helical termini.
Keywords: Scholl reaction; chiroptical; circulenes; dehydrohelicene; helicenes; material-based applications; nanographene; photophysical; quasicirculene; synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Chiroptical Amplification of [7]-Helicene Nanographene by Additional Helical Chirality.Angew Chem Int Ed Engl. 2025 Feb 3;64(6):e202420767. doi: 10.1002/anie.202420767. Epub 2024 Dec 18. Angew Chem Int Ed Engl. 2025. PMID: 39641263
-
Chasing Turns and Twists: Unraveling the One-Step Synthesis, Intricate Pathways, and Structural Revelations of N-Aryl Aza-quasi[8]circulenes.Chemistry. 2024 Jan 2;30(1):e202302876. doi: 10.1002/chem.202302876. Epub 2023 Nov 8. Chemistry. 2024. PMID: 37747146
-
Compressing a Non-Planar Aromatic Heterocyclic [7]Helicene to a Planar Hetero[8]Circulene.Chemistry. 2020 Apr 16;26(22):4935-4940. doi: 10.1002/chem.201905339. Epub 2020 Mar 20. Chemistry. 2020. PMID: 32052498
-
Helicene Radicals: Molecules Bearing a Combination of Helical Chirality and Unpaired Electron Spin.Chempluschem. 2020 Sep;85(9):2093-2104. doi: 10.1002/cplu.202000452. Chempluschem. 2020. PMID: 32935935 Review.
-
Scholl reaction as a powerful tool for the synthesis of nanographenes: a systematic review.RSC Adv. 2021 Sep 29;11(51):32158-32202. doi: 10.1039/d1ra05910f. eCollection 2021 Sep 27. RSC Adv. 2021. PMID: 35495486 Free PMC article. Review.
Cited by
-
Electrochemical cascade access to hetero[8]circulenes as potent organophotocatalysts for diverse C-X bond formations.Nat Commun. 2025 Jul 1;16(1):5682. doi: 10.1038/s41467-025-60889-w. Nat Commun. 2025. PMID: 40593683 Free PMC article.
References
-
- Pasteur L. Memoires sur la relation qui peut exister entre la forme crystalline et al. composition chimique, et sur la cause de la polarization rotatoire. Compt. Rend. 1848;26:535–538.
-
- van’t Hoff J.H. A suggestion looking to the extension into space of the structural formulas at present used in chemistry, and a note upon the relation between the optical activity and the chemical constitution of organic compounds. Arch. Neerl. Sci. Exactes Nat. 1874;9:445–454.
-
- Le Bel J.A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874;22:337–347.
-
- Cahn R.S., Ingold C., Prelog V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966;5:385–415. doi: 10.1002/anie.196603851. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

