A Two-Step Mechanism for Creating Stable, Condensed Chromatin with the Polycomb Complex PRC1
- PMID: 38257239
- PMCID: PMC10821450
- DOI: 10.3390/molecules29020323
A Two-Step Mechanism for Creating Stable, Condensed Chromatin with the Polycomb Complex PRC1
Abstract
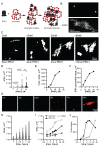
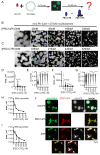
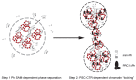
The Drosophila PRC1 complex regulates gene expression by modifying histone proteins and chromatin architecture. Two PRC1 subunits, PSC and Ph, are most implicated in chromatin architecture. In vitro, PRC1 compacts chromatin and inhibits transcription and nucleosome remodeling. The long disordered C-terminal region of PSC (PSC-CTR) is important for these activities, while Ph has little effect. In cells, Ph is important for condensate formation, long-range chromatin interactions, and gene regulation, and its polymerizing sterile alpha motif (SAM) is implicated in these activities. In vitro, truncated Ph containing the SAM and two other conserved domains (mini-Ph) undergoes phase separation with chromatin, suggesting a mechanism for SAM-dependent condensate formation in vivo. How the distinct activities of PSC and Ph on chromatin function together in PRC1 is not known. To address this question, we analyzed structures formed with large chromatin templates and PRC1 in vitro. PRC1 bridges chromatin into extensive fibrillar networks. Ph, its SAM, and SAM polymerization activity have little effect on these structures. Instead, the PSC-CTR controls their growth, and is sufficient for their formation. To understand how phase separation driven by Ph SAM intersects with the chromatin bridging activity of the PSC-CTR, we used mini-Ph to form condensates with chromatin and then challenged them with PRC1 lacking Ph (PRC1ΔPh). PRC1ΔPh converts mini-Ph chromatin condensates into clusters of small non-fusing condensates and bridged fibers. These condensates retain a high level of chromatin compaction and do not intermix. Thus, phase separation of chromatin by mini-Ph, followed by the action of the PSC-CTR, creates a unique chromatin organization with regions of high nucleosome density and extraordinary stability. We discuss how this coordinated sequential activity of two proteins found in the same complex may occur and the possible implications of stable chromatin architectures in maintaining transcription states.
Keywords: Polycomb; chromatin; intrinsically disordered region (IDR); phase separation biomolecular condensate; sterile alpha motif (SAM).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Regulation of Polyhomeotic Condensates by Intrinsically Disordered Sequences That Affect Chromatin Binding.Epigenomes. 2022 Nov 3;6(4):40. doi: 10.3390/epigenomes6040040. Epigenomes. 2022. PMID: 36412795 Free PMC article.
-
DNA Binding Reorganizes the Intrinsically Disordered C-Terminal Region of PSC in Drosophila PRC1.J Mol Biol. 2020 Aug 7;432(17):4856-4871. doi: 10.1016/j.jmb.2020.07.002. Epub 2020 Jul 3. J Mol Biol. 2020. PMID: 32628956 Free PMC article.
-
Phase separation by the polyhomeotic sterile alpha motif compartmentalizes Polycomb Group proteins and enhances their activity.Nat Commun. 2020 Nov 5;11(1):5609. doi: 10.1038/s41467-020-19435-z. Nat Commun. 2020. PMID: 33154383 Free PMC article.
-
Chromatin regulation: how complex does it get?Epigenetics. 2014 Nov;9(11):1485-95. doi: 10.4161/15592294.2014.971580. Epigenetics. 2014. PMID: 25482055 Free PMC article. Review.
-
Changing the DNA landscape: putting a SPN on chromatin.Curr Top Microbiol Immunol. 2003;274:171-201. doi: 10.1007/978-3-642-55747-7_7. Curr Top Microbiol Immunol. 2003. PMID: 12596908 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

