Phosphine Catalyzed Michael-Type Additions: The Synthesis of Glutamic Acid Derivatives from Arylidene- α-amino Esters
- PMID: 38257255
- PMCID: PMC10820836
- DOI: 10.3390/molecules29020342
Phosphine Catalyzed Michael-Type Additions: The Synthesis of Glutamic Acid Derivatives from Arylidene- α-amino Esters
Abstract
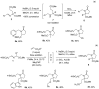
The reaction of arylidene-α-amino esters with electrophilic alkenes to yield Michael-type addition compounds is optimized using several phosphines as organocatalysts. The transformation is very complicated due to the generation of several final compounds, including those derived from the 1,3-dipolar cycloadditions. For this reason, the selection of the reaction conditions is a very complex task and the slow addition of the acrylic system is very important to complete the process. The study of the variation in the structural components of the starting imino ester is performed as well as the expansion of other electron-poor alkenes. The crude products have a purity higher than 90% in most cases without any purification. A plausible mechanism is detailed based on the bibliography and the experimental results. The synthesis of pyroglutamate entities, after the reduction of the imino group and cyclization, is performed in high yields. In addition, the hydrolysis of the imino group, under acidic media, represents a direct access to glutamate surrogates.
Keywords: Michael addition; glutamates; imino esters; organocatalysis; phosphines; pyroglutamates.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Eder I., Haider V., Zebrowski P., Waser M. Recent Progress in the Asymmetric Syntheses of α-Heterofunctionalized (Masked) α- and β-Amino Acid Derivatives. Eur. J. Org. Chem. 2021;2021:202–219. doi: 10.1002/ejoc.202001077. - DOI
-
- Shatskiy A., Kaerkaes M.D. Photoredox-enabled decarboxylative synthesis of unnatural α-amino acids. Synlett. 2022;33:109–115. doi: 10.1055/a-1499-8679. - DOI
Grants and funding
- RED2022-134287-T ORFEO CINQA and RED2022-134331-T CASI/Esciencia (Spain)
- projects CTQ2017-82935-P and PID2019-107268GB-I00/Ministerio de Economía, Industria y Competitividad
- GRISOLIAP/2020/111, IDIFEDER/2021/013, GVA-COVID19/2021/079 and CIDEGENT/2020/058/Generalitat Valenciana
- VIGROB-068, UAUSTI21-05/University of Alicante
- Medalchemy 22T/Medalchemy S.L.
LinkOut - more resources
Full Text Sources

