Aromatase Inhibitors as a Promising Direction for the Search for New Anticancer Drugs
- PMID: 38257259
- PMCID: PMC10819800
- DOI: 10.3390/molecules29020346
Aromatase Inhibitors as a Promising Direction for the Search for New Anticancer Drugs
Abstract
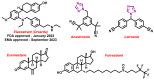
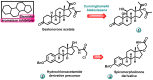
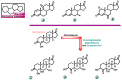
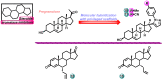
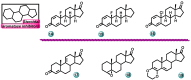
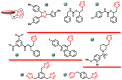
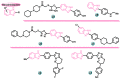
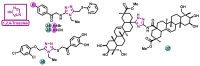
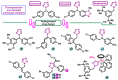
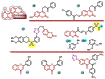
Aromatase is an enzyme that plays a crucial role in the biosynthesis of estrogens, which are hormones that contribute to the growth of certain types of breast cancer. In particular, aromatase catalyzes the conversion of androgens (male hormones) into estrogens (female hormones) in various tissues, including the adrenal glands, ovaries, and adipose tissue. Given the role of estrogen in promoting the growth of hormone-receptor-positive breast cancers, aromatase has become an important molecular target for the development of anticancer agents. Aromatase inhibitors can be classified into two main groups based on their chemical structure: steroidal and non-steroidal inhibitors. This work presents a review of the literature from the last ten years regarding the search for new aromatase inhibitors. We present the directions of search, taking into account the impact of structure modifications on anticancer activity.
Keywords: anticancer activity; aromatase inhibitors; steroidal and non-steroidal compounds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
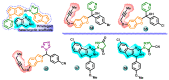
Similar articles
-
Recent developments in steroidal and nonsteroidal aromatase inhibitors for the chemoprevention of estrogen-dependent breast cancer.Eur J Med Chem. 2015 Sep 18;102:375-86. doi: 10.1016/j.ejmech.2015.08.010. Epub 2015 Aug 8. Eur J Med Chem. 2015. PMID: 26301554
-
Aromatase in the context of breast and endometrial cancer. A review.Minerva Endocrinol. 2006 Mar;31(1):47-60. Minerva Endocrinol. 2006. PMID: 16498363 Review.
-
Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer.Semin Oncol. 2003 Aug;30(4 Suppl 14):3-11. doi: 10.1016/s0093-7754(03)00302-6. Semin Oncol. 2003. PMID: 14513432 Review.
-
Dual Aromatase-Sulphatase Inhibitors (DASIs) for the Treatment of Hormone Dependent Breast Cancer.Mini Rev Med Chem. 2021;21(20):3144-3165. doi: 10.2174/1389557521666210119123840. Mini Rev Med Chem. 2021. PMID: 33463462 Review.
-
Effects of new C6-substituted steroidal aromatase inhibitors in hormone-sensitive breast cancer cells: Cell death mechanisms and modulation of estrogen and androgen receptors.J Steroid Biochem Mol Biol. 2019 Dec;195:105486. doi: 10.1016/j.jsbmb.2019.105486. Epub 2019 Sep 23. J Steroid Biochem Mol Biol. 2019. PMID: 31557516
Cited by
-
(E)-1-(3-(3-Hydroxy-4-Methoxyphenyl)-1-(3,4,5-Trimethoxyphenyl)allyl)-1H-1,2,4-Triazole and Related Compounds: Their Synthesis and Biological Evaluation as Novel Antimitotic Agents Targeting Breast Cancer.Pharmaceuticals (Basel). 2025 Jan 17;18(1):118. doi: 10.3390/ph18010118. Pharmaceuticals (Basel). 2025. PMID: 39861179 Free PMC article.
-
Current Endocrine Therapy in Hormone-Receptor-Positive Breast Cancer: From Tumor Biology to the Rationale for Therapeutic Tunning.Medicina (Kaunas). 2025 Jul 16;61(7):1280. doi: 10.3390/medicina61071280. Medicina (Kaunas). 2025. PMID: 40731911 Free PMC article. Review.
-
Current Evidence on the Impact of Diet, Food, and Supplement Intake on Breast Cancer Health Outcomes in Patients Undergoing Endocrine Therapy.Nutrients. 2025 Jan 26;17(3):456. doi: 10.3390/nu17030456. Nutrients. 2025. PMID: 39940314 Free PMC article. Review.
References
-
- Sayyad N.B., Sabale P.M., Umare M.D., Bajaj K.K. Aromatase Inhibitors: Development and Current Perspectives. Indian J. Pharm. Educ. Res. 2022;56:311–320. doi: 10.5530/ijper.56.2.51. - DOI
-
- Çetiner G., Acar Çevik U., Celik I., Bostancı H.E., Özkay Y., Kaplancıklı Z.A. New Imidazole Derivatives as Aromatase Inhibitor: Design, Synthesis, Biological Activity, Molecular Docking, and Computational ADME-Tox Studies. J. Mol. Struct. 2023;1278:134920. doi: 10.1016/j.molstruc.2023.134920. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

