Enzymatic Protein Immobilization for Nanobody Array
- PMID: 38257279
- PMCID: PMC10820937
- DOI: 10.3390/molecules29020366
Enzymatic Protein Immobilization for Nanobody Array
Abstract
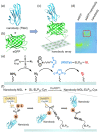
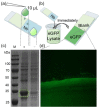
Antibody arrays play a pivotal role in the detection and quantification of biomolecules, with their effectiveness largely dependent on efficient protein immobilization. Traditional methods often use heterobifunctional cross-linking reagents for attaching functional residues in proteins to corresponding chemical groups on the substrate surface. However, this method does not control the antibody's anchoring point and orientation, potentially leading to reduced binding efficiency and overall performance. Another method using anti-antibodies as intermediate molecules to control the orientation can be used but it demonstrates lower efficiency. Here, we demonstrate a site-specific protein immobilization strategy utilizing OaAEP1 (asparaginyl endopeptidase) for building a nanobody array. Moreover, we used a nanobody-targeting enhanced green fluorescent protein (eGFP) as the model system to validate the protein immobilization method for building a nanobody array. Finally, by rapidly enriching eGFP, this method further highlights its potential for rapid diagnostic applications. This approach, characterized by its simplicity, high efficiency, and specificity, offers an advancement in the development of surface-modified protein arrays. It promises to enhance the sensitivity and accuracy of biomolecule detection, paving the way for broader applications in various research and diagnostic fields.
Keywords: OaAEP1; enzymatic ligation; nanobody; protein immobilization.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Nanobody‑horseradish peroxidase and -EGFP fusions as reagents to detect porcine parvovirus in the immunoassays.J Nanobiotechnology. 2020 Jan 7;18(1):7. doi: 10.1186/s12951-019-0568-x. J Nanobiotechnology. 2020. PMID: 31910833 Free PMC article.
-
One-step asparaginyl endopeptidase (OaAEP1)-based protein immobilization for single-molecule force spectroscopy.RSC Chem Biol. 2022 Aug 30;3(10):1276-1281. doi: 10.1039/d2cb00135g. eCollection 2022 Oct 5. RSC Chem Biol. 2022. PMID: 36320890 Free PMC article.
-
Nanobody-horseradish peroxidase fusion protein as an ultrasensitive probe to detect antibodies against Newcastle disease virus in the immunoassay.J Nanobiotechnology. 2019 Mar 1;17(1):35. doi: 10.1186/s12951-019-0468-0. J Nanobiotechnology. 2019. PMID: 30823927 Free PMC article.
-
A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers.Nanomaterials (Basel). 2020 Oct 27;10(11):2142. doi: 10.3390/nano10112142. Nanomaterials (Basel). 2020. PMID: 33121181 Free PMC article. Review.
-
Site-selective orientated immobilization of antibodies and conjugates for immunodiagnostics development.Methods. 2017 Mar 1;116:95-111. doi: 10.1016/j.ymeth.2016.11.010. Epub 2016 Nov 19. Methods. 2017. PMID: 27876681 Free PMC article. Review.
References
-
- Bergsma D., Chen S.M., Buchweitz J., Gerszten R., Haab B.B. Antibody-array interaction mapping, a new method to detect protein complexes applied to the discovery and study of serum amyloid P interactions with kininogen in human plasma. Mol. Cell Proteom. 2010;9:446–456. doi: 10.1074/mcp.M900418-MCP200. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

