Recent Developments in Synthesis, Properties, Applications and Recycling of Bio-Based Elastomers
- PMID: 38257300
- PMCID: PMC10819226
- DOI: 10.3390/molecules29020387
Recent Developments in Synthesis, Properties, Applications and Recycling of Bio-Based Elastomers
Abstract
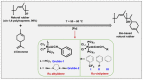
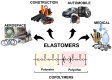
In 2021, global plastics production was 390.7 Mt; in 2022, it was 400.3 Mt, showing an increase of 2.4%, and this rising tendency will increase yearly. Of this data, less than 2% correspond to bio-based plastics. Currently, polymers, including elastomers, are non-recyclable and come from non-renewable sources. Additionally, most elastomers are thermosets, making them complex to recycle and reuse. It takes hundreds to thousands of years to decompose or biodegrade, contributing to plastic waste accumulation, nano and microplastic formation, and environmental pollution. Due to this, the synthesis of elastomers from natural and renewable resources has attracted the attention of researchers and industries. In this review paper, new methods and strategies are proposed for the preparation of bio-based elastomers. The main goals are the advances and improvements in the synthesis, properties, and applications of bio-based elastomers from natural and industrial rubbers, polyurethanes, polyesters, and polyethers, and an approach to their circular economy and sustainability. Olefin metathesis is proposed as a novel and sustainable method for the synthesis of bio-based elastomers, which allows for the depolymerization or degradation of rubbers with the use of essential oils, terpenes, fatty acids, and fatty alcohols from natural resources such as chain transfer agents (CTA) or donors of the terminal groups in the main chain, which allow for control of the molecular weights and functional groups, obtaining new compounds, oligomers, and bio-based elastomers with an added value for the application of new polymers and materials. This tendency contributes to the development of bio-based elastomers that can reduce carbon emissions, avoid cross-contamination from fossil fuels, and obtain a greener material with biodegradable and/or compostable behavior.
Keywords: bio-based elastomer; circular economy; elastomeric properties; olefin metathesis; polyester; polyether; polyurethane; rubbers; sustainability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Plastics Europe AISBL Plastics-the Facts 2022. [(accessed on 17 November 2023)]. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/
-
- Plastics Europe AISBL Plastics-the Fast Facts 2023. [(accessed on 28 November 2023)]. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/
-
- Aiswarya S., Awasthi P., Banerjee S.S. Self-Healing Thermoplastic Elastomeric Materials: Challenges, Opportunities and New Approaches. Eur. Polym. J. 2022;181:111658. doi: 10.1016/j.eurpolymj.2022.111658. - DOI
-
- Tang S., Li J., Wang R., Zhang J., Lu Y., Hu G., Wang Z., Zhang L. Current Trends in Bio-based Elastomer Materials. SusMat. 2022;2:2–33. doi: 10.1002/sus2.45. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

