Sequential Immunizations with Influenza Neuraminidase Protein Followed by Peptide Nanoclusters Induce Heterologous Protection
- PMID: 38257777
- PMCID: PMC10819419
- DOI: 10.3390/v16010077
Sequential Immunizations with Influenza Neuraminidase Protein Followed by Peptide Nanoclusters Induce Heterologous Protection
Abstract
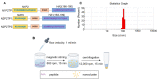
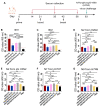
Enhancing cross-protections against diverse influenza viruses is desired for influenza vaccinations. Neuraminidase (NA)-specific antibody responses have been found to independently correlate with a broader influenza protection spectrum. Here, we report a sequential immunization regimen that includes priming with NA protein followed by boosting with peptide nanoclusters, with which targeted enhancement of antibody responses in BALB/c mice to certain cross-protective B-cell epitopes of NA was achieved. The nanoclusters were fabricated via desolvation with absolute ethanol and were only composed of composite peptides. Unlike KLH conjugates, peptide nanoclusters would not induce influenza-unrelated immunity. We found that the incorporation of a hemagglutinin peptide of H2-d class II restriction into the composite peptides could be beneficial in enhancing the NA peptide-specific antibody response. Of note, boosters with N2 peptide nanoclusters induced stronger serum cross-reactivities to heterologous N2 and even heterosubtypic N7 and N9 than triple immunizations with the prototype recombinant tetrameric (rt) N2. The mouse challenge experiments with HK68 H3N2 also demonstrated the strong effectiveness of the peptide nanocluster boosters in conferring heterologous protection.
Keywords: cross-protection; influenza vaccine; nanocluster; neuraminidase; sequential immunization; serum cross-reactivity.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Boost immunizations with NA-derived peptide conjugates achieve induction of NA inhibition antibodies and heterologous influenza protections.Cell Rep. 2023 Jul 25;42(7):112766. doi: 10.1016/j.celrep.2023.112766. Epub 2023 Jul 7. Cell Rep. 2023. PMID: 37421618
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Cross-Reactive Neuraminidase-Inhibiting Antibodies Elicited by Immunization with Recombinant Neuraminidase Proteins of H5N1 and Pandemic H1N1 Influenza A Viruses.J Virol. 2015 Jul;89(14):7224-34. doi: 10.1128/JVI.00585-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948745 Free PMC article.
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
References
-
- Impagliazzo A., Milder F., Kuipers H., Wagner M.V., Zhu X., Hoffman R.M., van Meersbergen R., Huizingh J., Wanningen P., Verspuij J., et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349:1301–1306. - PubMed
-
- Stadlbauer D., Zhu X., McMahon M., Turner J.S., Wohlbold T.J., Schmitz A.J., Strohmeier S., Yu W., Nachbagauer R., Mudd P.A., et al. Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science. 2019;366:499–504. doi: 10.1126/science.aay0678. - DOI - PMC - PubMed
-
- Madsen A., Dai Y.N., McMahon M., Schmitz A.J., Turner J.S., Tan J., Lei T., Alsoussi W.B., Strohmeier S., Amor M., et al. Human Antibodies Targeting Influenza B Virus Neuraminidase Active Site Are Broadly Protective. Immunity. 2020;53:852–863 e7. doi: 10.1016/j.immuni.2020.08.015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

