Exploring Paxlovid Efficacy in COVID-19 Patients with MAFLD: Insights from a Single-Center Prospective Cohort Study
- PMID: 38257811
- PMCID: PMC10819977
- DOI: 10.3390/v16010112
Exploring Paxlovid Efficacy in COVID-19 Patients with MAFLD: Insights from a Single-Center Prospective Cohort Study
Abstract
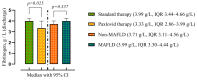
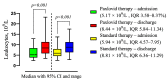
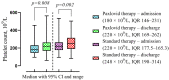
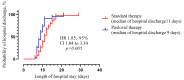
This study investigates the intricate interplay between Metabolic-associated Fatty Liver Disease (MAFLD) and COVID-19, exploring the impact of MAFLD on disease severity, outcomes, and the efficacy of the antiviral agent Paxlovid (nirmatrelvir/ritonavir). MAFLD, affecting a quarter of the global population, emerges as a potential risk factor for severe COVID-19, yet the underlying pathophysiological mechanisms remain elusive. This study focuses on the clinical significance of Paxlovid, the first orally bioavailable antiviral agent granted Emergency Use Authorization in the United States. Notably, outcomes from phase II/III trials exhibit an 88% relative risk reduction in COVID-19-associated hospitalization or mortality among high-risk patients. Despite conflicting data on the association between MAFLD and COVID-19 severity, this research strives to bridge the gap by evaluating the effectiveness of Paxlovid in MAFLD patients with COVID-19, addressing the scarcity of relevant studies.
Keywords: COVID-19; MAFLD; Paxlovid; nirmatrelvir.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Yoo H.W., Jin H.Y., Yon D.K., Effenberger M., Shin Y.H., Kim S.Y., Yang J.M., Kim M.S., Koyanagi A., Jacob L., et al. Non-Alcoholic Fatty Liver Disease and COVID-19 Susceptibility and Outcomes: A Korean Nationwide Cohort. J. Korean Med. Sci. 2021;36:e291. doi: 10.3346/jkms.2021.36.e291. - DOI - PMC - PubMed
-
- Eslam M., Sanyal A.J., George J., Sanyal A., Neuschwander-Tetri B., Tiribelli C., Kleiner D.E., Brunt E., Bugianesi E., Yki-Järvinen H., et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. - DOI - PubMed
-
- Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wai-Sun Wong V., Dufour J.-F., Schattenberg J.M., et al. A New Definition for Metabolic Dysfunction-Associated Fatty Liver Disease: An International Expert Consensus Statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. - DOI - PubMed
-
- Forlano R., Mullish B.H., Mukherjee S.K., Nathwani R., Harlow C., Crook P., Judge R., Soubieres A., Middleton P., Daunt A., et al. In-Hospital Mortality Is Associated with Inflammatory Response in NAFLD Patients Admitted for COVID-19. PLoS ONE. 2020;15:e0240400. doi: 10.1371/journal.pone.0240400. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

