Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK
- PMID: 38257840
- PMCID: PMC10819102
- DOI: 10.3390/v16010140
Retinoic Acid-Mediated Inhibition of Mouse Coronavirus Replication Is Dependent on IRF3 and CaMKK
Abstract
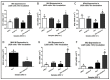
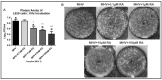
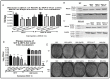
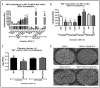
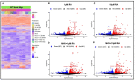
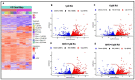
The ongoing COVID-19 pandemic has revealed the shortfalls in our understanding of how to treat coronavirus infections. With almost 7 million case fatalities of COVID-19 globally, the catalog of FDA-approved antiviral therapeutics is limited compared to other medications, such as antibiotics. All-trans retinoic acid (RA), or activated vitamin A, has been studied as a potential therapeutic against coronavirus infection because of its antiviral properties. Due to its impact on different signaling pathways, RA's mechanism of action during coronavirus infection has not been thoroughly described. To determine RA's mechanism of action, we examined its effect against a mouse coronavirus, mouse hepatitis virus strain A59 (MHV). We demonstrated that RA significantly decreased viral titers in infected mouse L929 fibroblasts and RAW 264.7 macrophages. The reduced viral titers were associated with a corresponding decrease in MHV nucleocapsid protein expression. Using interferon regulatory factor 3 (IRF3) knockout RAW 264.7 cells, we demonstrated that RA-induced suppression of MHV required IRF3 activity. RNA-seq analysis of wildtype and IRF3 knockout RAW cells showed that RA upregulated calcium/calmodulin (CaM) signaling proteins, such as CaM kinase kinase 1 (CaMKK1). When treated with a CaMKK inhibitor, RA was unable to upregulate IRF activation during MHV infection. In conclusion, our results demonstrate that RA-induced protection against coronavirus infection depends on IRF3 and CaMKK.
Keywords: CaMKK; IRF3; MHV; coronavirus; retinoic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

