The Evolving Microbiome of Dental Caries
- PMID: 38257948
- PMCID: PMC10819217
- DOI: 10.3390/microorganisms12010121
The Evolving Microbiome of Dental Caries
Abstract
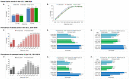
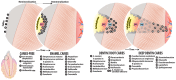
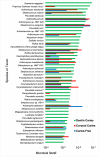
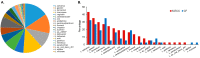
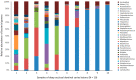
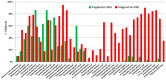
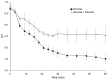
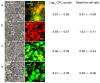
Dental caries is a significant oral and public health problem worldwide, especially in low-income populations. The risk of dental caries increases with frequent intake of dietary carbohydrates, including sugars, leading to increased acidity and disruption of the symbiotic diverse and complex microbial community of health. Excess acid production leads to a dysbiotic shift in the bacterial biofilm composition, demineralization of tooth structure, and cavities. Highly acidic and acid-tolerant species associated with caries include Streptococcus mutans, Lactobacillus, Actinomyces, Bifidobacterium, and Scardovia species. The differences in microbiotas depend on tooth site, extent of carious lesions, and rate of disease progression. Metagenomics and metatranscriptomics not only reveal the structure and genetic potential of the caries-associated microbiome, but, more importantly, capture the genetic makeup of the metabolically active microbiome in lesion sites. Due to its multifactorial nature, caries has been difficult to prevent. The use of topical fluoride has had a significant impact on reducing caries in clinical settings, but the approach is costly; the results are less sustainable for high-caries-risk individuals, especially children. Developing treatment regimens that specifically target S. mutans and other acidogenic bacteria, such as using nanoparticles, show promise in altering the cariogenic microbiome, thereby combatting the disease.
Keywords: Streptococcus mutans; caries; ecology; microbiology; treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Milgrom P. Tooth decay in our poorest children: What can we do? J. Indiana Dent. Assoc. 2000;79:24–26. - PubMed
-
- GBD 2017 Oral Disorders Collaborators. Bernabe E., Marcenes W., Hernandez C.R., Bailey J., Abreu L.G., Alipour V., Amini S., Arabloo J., Arefi Z., et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: A systematic analysis for the global burden of disease 2017 study. J. Dent. Res. 2020;99:362–373. doi: 10.1177/0022034520908533. - DOI - PMC - PubMed
-
- Agency for Healthcare Research and Quality . MEPS HC-188B: 2016 Dental Visits. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2018.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

