Gut Microbiome Disruption Following SARS-CoV-2: A Review
- PMID: 38257958
- PMCID: PMC10820238
- DOI: 10.3390/microorganisms12010131
Gut Microbiome Disruption Following SARS-CoV-2: A Review
Abstract
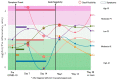
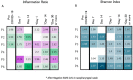
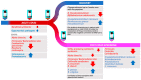
COVID-19 has been associated with having a negative impact on patients' gut microbiome during both active disease and in the post-acute phase. In acute COVID-19, rapid alteration of the gut microbiome composition was observed, showing on one side a reduction in beneficial symbionts (e.g., Roseburia, Lachnospiraceae) and on the other side an increase in opportunistic pathogens such as Enterococcus and Proteobacteria. Alpha diversity tends to decrease, especially initially with symptom onset and hospital admission. Although clinical recovery appears to align with improved gut homeostasis, this process could take several weeks, even in mild infections. Moreover, patients with COVID-19 post-acute syndrome showed changes in gut microbiome composition, with specific signatures associated with decreased respiratory function up to 12 months following acute disease. Potential treatments, especially probiotic-based therapy, are under investigation. Open questions remain on the possibility to use gut microbiome data to predict disease progression and on potential confounders that may impair result interpretation (e.g., concomitant therapies in the acute phase; reinfection, vaccines, and occurrence of novel conditions or diseases in the post-acute syndrome). Understanding the relationships between gut microbiome dynamics and disease progression may contribute to better understanding post-COVID syndrome pathogenesis or inform personalized treatment that can affect specific targets or microbiome markers.
Keywords: COVID-19; beneficial symbionts; microbiome; opportunistic pathogens; post-COVID-19 syndrome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zuo T., Zhan H., Zhang F., Liu Q., Tso E.Y.K., Lui G.C.Y., Chen N., Li A., Lu W., Francis K., et al. Alterations in Fecal Fungal Microbiome of Patients With COVID-19 During Time of Hospitalization until Discharge. Gastroenterology. 2020;159:1302–1310.e5. doi: 10.1053/j.gastro.2020.06.048. - DOI - PMC - PubMed
-
- Post COVID-19 Condition (Long COVID) [Internet] [(accessed on 3 October 2023)]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-cond....
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

